Obtaining the right balance between protein purity and yield of purified protein is key to a successful multistep purification protocol. This article shows you how to combine chromatography techniques to optimize your purification protocol when purifying untagged and native proteins
Untagged protein purification vs tagged protein purification
Most proteins purified in laboratory scale are affinity tagged and can therefore be purified with relative ease using affinity chromatography (AC). However, in some instances, the protein to be purified is untagged (also called nontagged), for the following reasons:
- The untagged protein comes from a natural source (native protein).
- The untagged protein is a recombinant protein that has been overexpressed without a tag, which would otherwise interfere with the protein structure or activity.
Several reliable approaches to purification of untagged proteins are available.
What to consider when planning your untagged protein purification experiment
Untagged proteins usually require a multistep purification protocol.
- The choice of chromatography technique(s) depends on the purity requirement and yield of your protein of interest (Fig 1). The required protein purity level will depend on your application, as shown in the table below.
- Before you start, carefully define your objectives and consider that in general, every added purification step will increase purity but decrease total protein recovery1 and yield2.
1 Recovery = The relative amount of target protein (%) that is retrieved after purification compared with amount loaded on the column.
2 Yield = Amount of target protein obtained after a purification step, or after the entire purification (multiple steps).
| Typical applications | Purity level |
|---|---|
| Mass spectrometry Antigen for immunization | Moderate, > 80% |
| Functional studies Structural studies | Very high, 95% to 99% |
| Structural studies Therapeutic proteins | Highest, > 99% |
How to combine chromatography techniques to obtain a powerful untagged protein purification protocol
Untagged proteins can usually be sufficiently purified by combining purification methods that separate on the basis of different physicochemical characteristics of the proteins (orthogonal methods).
Figure 1 describes three typical proven chromatography technique combinations for the purification of untagged proteins.
2-step purification protocol: IEX + SEC
This purification protocol is a good starting point for most applications. It utilizes a first ion exchange chromatography (IEX) step, which is followed by a size exclusion chromatography (SEC)3 step. The end purity will be moderate (>80%), while the yield will be high.
3 Also called “gel filtration”
3-step purification protocols: two main options
The 3-step protocols using orthogonal techniques give the best purity (95% to 99%), while the yield will be moderate.
- Option 1: combination of IEX-HIC-SEC The combination IEX-HIC4-SEC three-step purification protocol is frequently used because the high-salt conditions after the first step can simply be adjusted with additional salt for HIC purification and followed by SEC for polishing and salt removal.
4 HIC = hydrophobic interaction chromatography
- Option 2: combination of HIC-IEX-SEC Option 2 is a good approach if ammonium sulfate precipitation has been used as a first sample concentration step.
Why use ammonium sulfate precipitation in the 3-step protocol?
Ammonium sulfate is sometimes used for initial sample concentration and cleanup. It stabilizes proteins without denaturation. The sample will contain a high salt concentration and may be applied directly to a HIC column with little or no additional preparation. If ammonium sulfate precipitation has been performed, the combination of HIC-IEX-SEC is suitable because HIC requires high-salt conditions for binding and gives elution in a relatively low salt concentration in a significantly smaller volume. Dilution or desalting can then be used to remove remaining salt, so that the sample can be bound to an IEX column.
Other multistep purification protocols might be used for purifying untagged proteins
The combinations given here will be efficient for most protein purifications if selected and optimized properly. Other combinations not given here or combinations that include more than three steps may be necessary in some instances.
What about combining IEX techniques?
IEX is a method that offers different selectivity by using either cation (CIEX) or anion (AIEX) exchangers. A purification protocol can also be designed to include a combination of CIEX and AIEX. The order of the IEX columns is dependent on the isoelectric point (pI) of the target protein and which contaminants you want to remove. Consider using the first column for binding and concentration of the target protein and the second column for binding of remaining contaminants (target protein flows through).
Which chromatography columns are recommended for each step?
Click on the blue buttons for recommended columns for each chromatography step
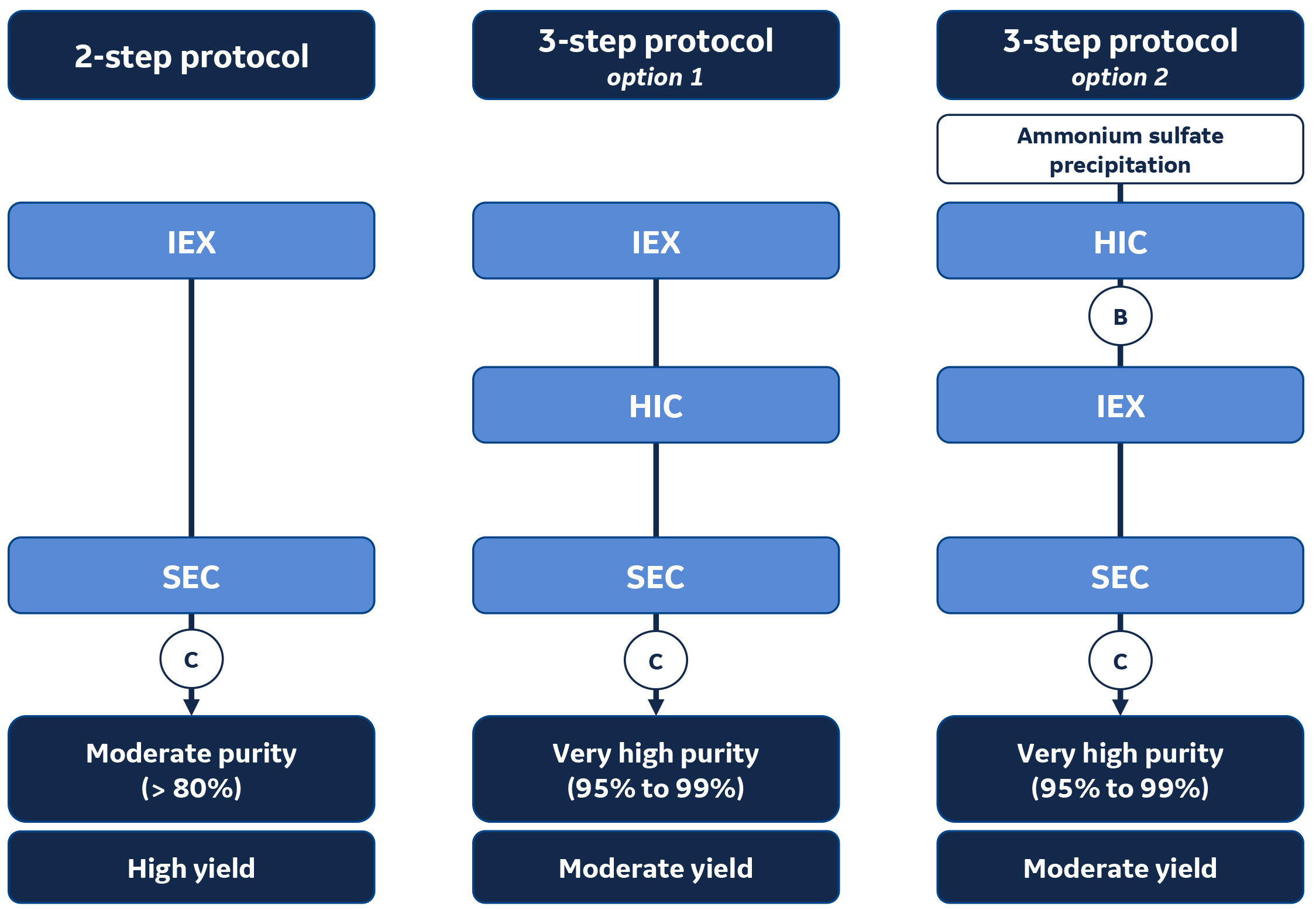
Fig 1. 2- or 3-step protocols may be deployed to purify untagged proteins depending on the goal of the purification (yield vs purity) —IEX = ion exchange chromatography; HIC = hydrophobic interaction chromatography; SEC = size exclusion chromatography; B = Buffer exchange; C = Concentration; Steps in circles are optional and are applied if necessary.
Importance of buffer exchange and concentration in the protein purification protocol
To make the sample compatible with the following steps or experiments, it might be necessary to use a desalting column for buffer exchange, and/or a concentration unit to reduce the sample volume.
This is what is represented by B and C in Figure 1.
- B: Buffer exchange to prepare for IEX.
- C: Concentration for sample volume reduction. May also be performed before SEC.
Automate multistep purification protocol to save time and add capacity
If you are using an ÄKTA pure system or an ÄKTA avant system, there is a way to set up your system to let it run the full sequence of chromatography runs on its own.
Read about how to automate multistep purification with ÄKTA systems
Purifying other types of proteins?
Read about how to combine chromatography techniques to purify antibodies or histagged proteins.