Challenges
Many CAR T cell manufacturing processes rely on manual and open unit operations that pose risks to commercial production. There is a need in the cell and gene therapy industry to reduce labor, manufacturing timelines, and costs while ensuring GMP compliance.
Developed process
Process development efforts delivered a semi-automated, closed CAR T cell process for commercial production using lentiviral transduction. The developed platform process is robust and flexible to suit the varied needs of CAR T developers. It is amenable to good manufacturing practices (GMP) compliance.
Outcomes
The developed process achieved:
- An average of > 78-fold cell expansion and > 1.0 x 1010 transduced T cells in 8 days.
- Greater than 80% transduction efficiency of enhanced green fluorescent protein (eGFP) using a lentiviral vector.
- Largely automated and closed process with a modular platform that is flexible to meet different needs.
Demand for improved CAR T manufacturing
With clinical successes of chimeric antigen receptor T (CAR T) cell therapies, there is demand for improvements in the CAR T manufacturing workflow that can be widely adopted by the cell and gene therapy industry. Currently, CAR T workflows rely on multiple manual, open, and lengthy unit operations. In this study we developed a robust CAR T cell production process using equipment and reagents that support closed processing. The process uses automation to reduce production costs and improve process consistency, while offering the flexibility to adapt to the varied needs of CAR T developers.
Methods for process development
Figure 1 shows the workflow of the developed process. The process started with a frozen leukopak and ended at day 8 with a cryopreserved product in a cryobag.
Fig 1. Workflow for the developed semi-automated, closed CAR T cell manufacturing process. The cell material at the start and end of the process is highlighted in gray. The unit operations in between are highlighted in white.
Results and discussion
At the start of the study, we selected instruments and reagents to support closed processing and automation across the workflow. Options were systematically tested to identify robust solutions for good manufacturing practices (GMP) manufacture of CAR T cells.
Leukopak thawing and PBMC isolation
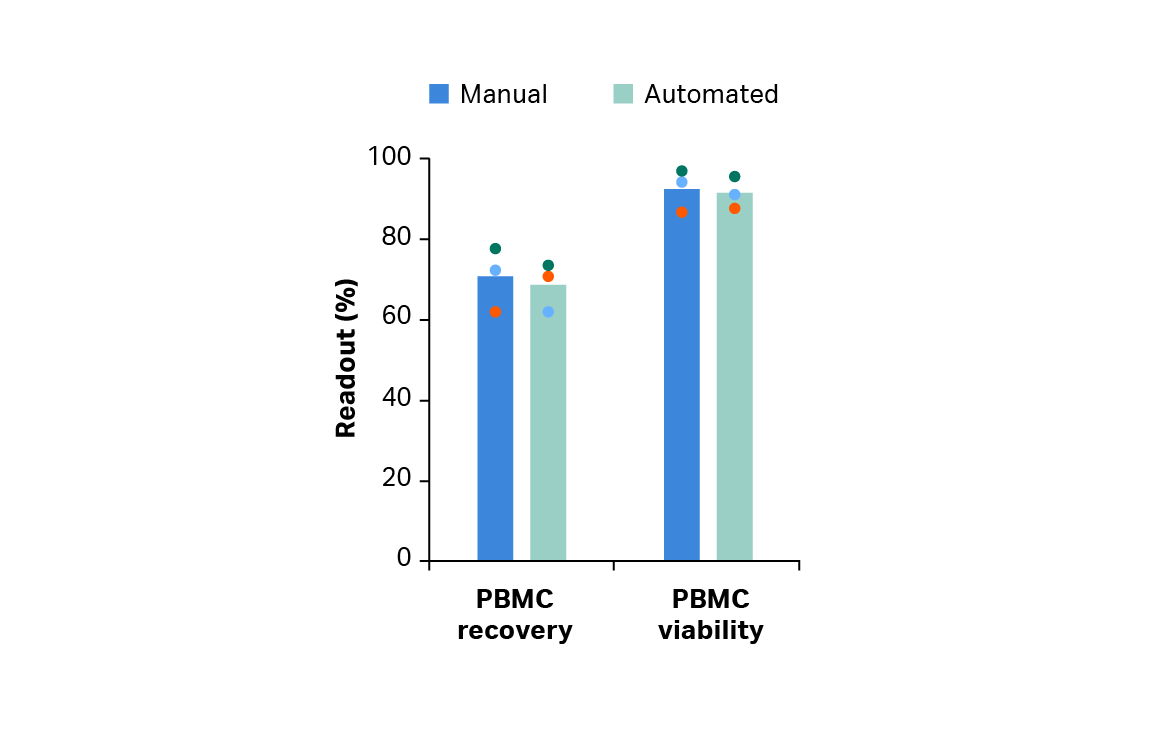
The Smart-Max device* and Sepax 2 system† were chosen for controlled leukopak thaw and peripheral blood mononuclear cell (PBMC) isolation, respectively. When paired, Smart-Max and Sepax 2 showed equivalent PBMC recovery and viability compared with manual methods (Fig 2).
* For upstream thawing, we used the Thaw-Large Volume Protocol Software on the Smart-Max instrument. An alternative would be to use the VIA Thaw instrument for both upstream and downstream processing. Thaw-Large Volume Protocol Software is coming soon. There is no guarantee regarding the release of any products (we reserve the right to change plans and timing in regards to the release of any products).
† Sepax 2 Cell Separation Device was used in this study, however the Sepax C-Pro is recommended as the equivalent instrument for manufacturing purposes.
Fig 2. Viable peripheral blood mononuclear cell (PBMC) percentage recovery and viability immediately after manual or automated thaw and wash. Bars represent the average of biological triplicates with individual donor values shown by colored dots.
T cell enrichment, activation, and lentiviral transduction
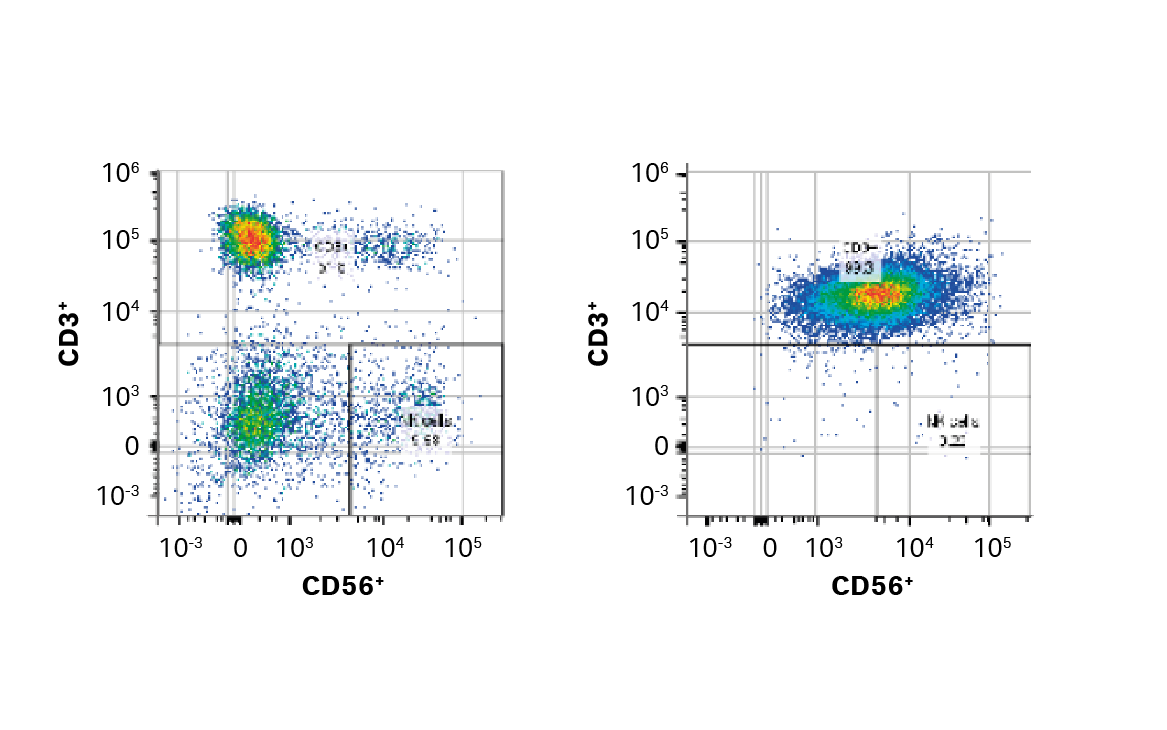
Three T cell enrichment methods were assessed. Of these, we chose EasySep™ Release Human CD3 Positive Selection (STEMCELL Technologies), which provided > 99% purity (Fig 3), equivalent viability and expansion metrics on day 5, and equivalent or superior recovery and purity compared to the other methods tested (recovery was significantly higher than other positive selection method; purity was significantly higher than the negative depletion method, p < 0.05).
Fig 3. FlowJo™ representation of gated cells before and after EasySep CD3+ selection.
To maintain process closure over the first four to five days of T cell culture, we identified a closed shake-flask platform. This platform showed comparable T cell growth, viability, and phenotype to manual T-flask cultures.
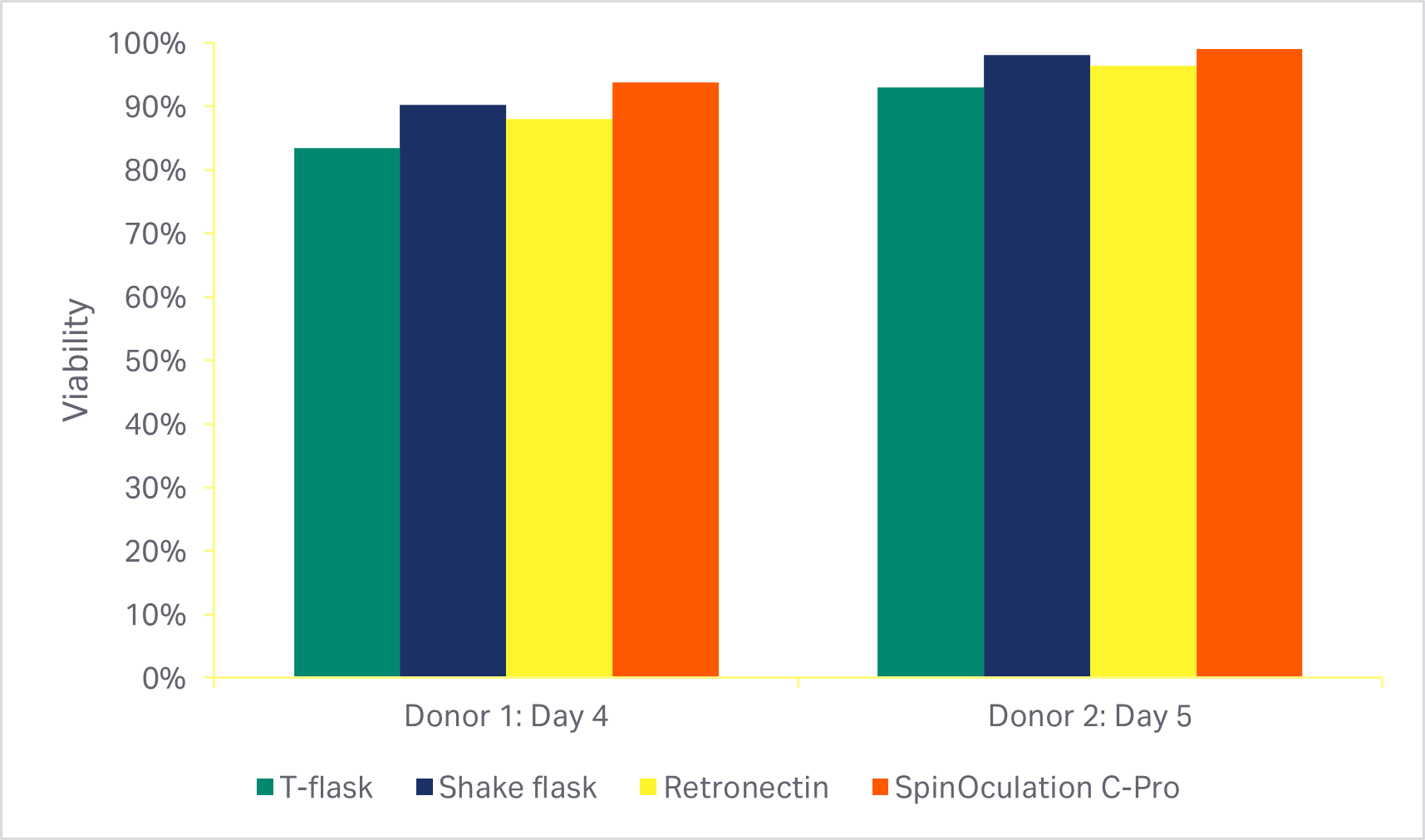
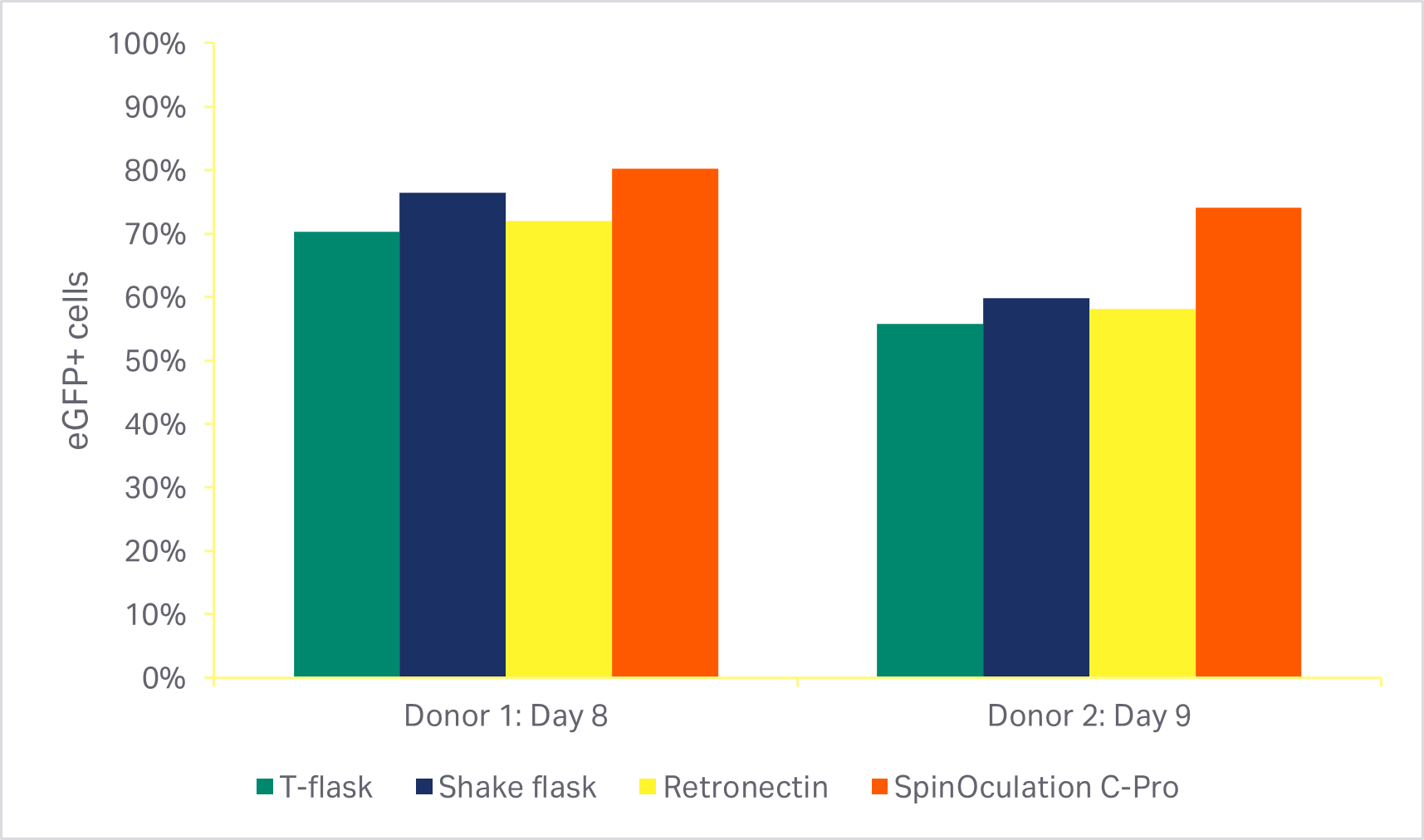
An automated, closed transduction method using the Sepax C-Pro system and SpinOculation C-Pro protocol software showed > 80% T cell transduction efficiency using research-grade eGFP lentivirus (Fig 4). Viability and transduction efficiency were comparable with three other commonly used methods, including RetroNectin™ coated plates.
(A)
(B)
Fig 4. Comparison of transduction methods, with MOI of 2.5. (A) Viability of cells using various transduction methods after activation phase of culture (3 days or 4 days after transduction); (B) percentage of eGFP-positive T cells at the end of culture(7 or 8 days after transduction). Bars represent the average of technical triplicates that were run for each of two biological donors.
T cell expansion
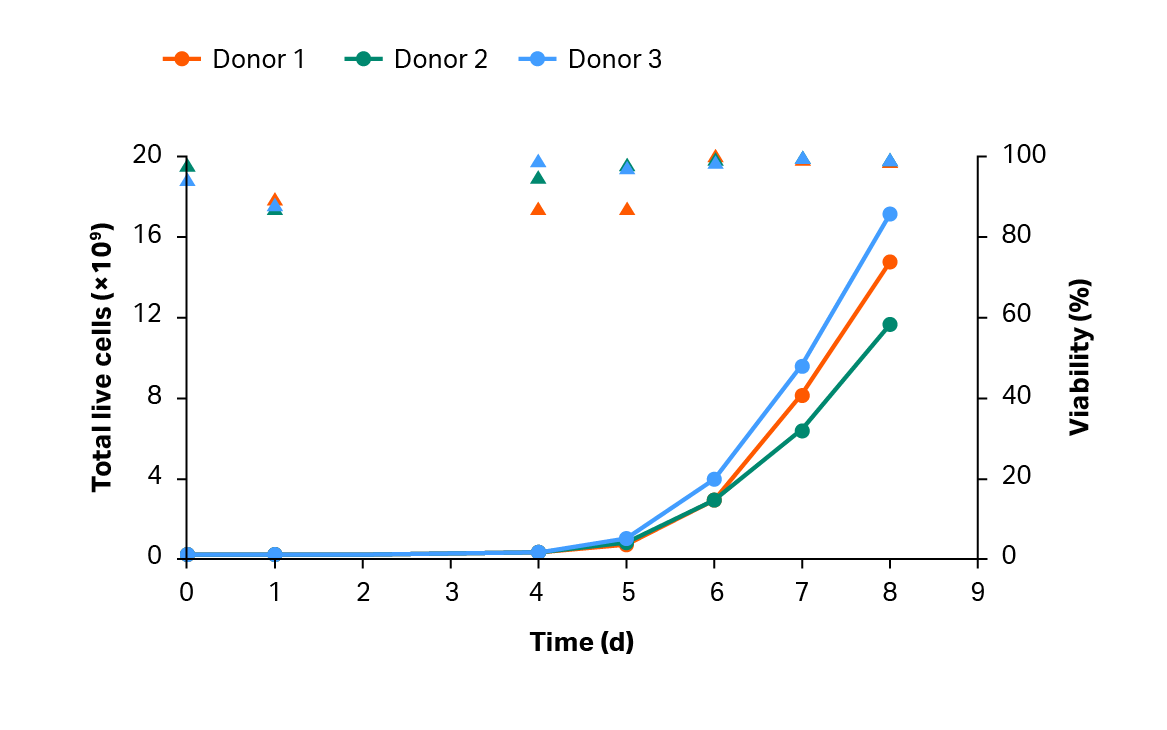
To further decrease labor and process variability, we devised a closed and controlled expansion strategy. In three large-scale production runs, transduced T cells were expanded in a Xuri W25 Cell Expansion System (Fig 5). The yield was > 1 × 1010 transduced T cells on day 8 of the process (Fig 6).
Fig 5. Cumulative growth and viability of T cells. Lines represent the expansion profile, and triangles represent the viability during the CAR T process.
(A)
(B)
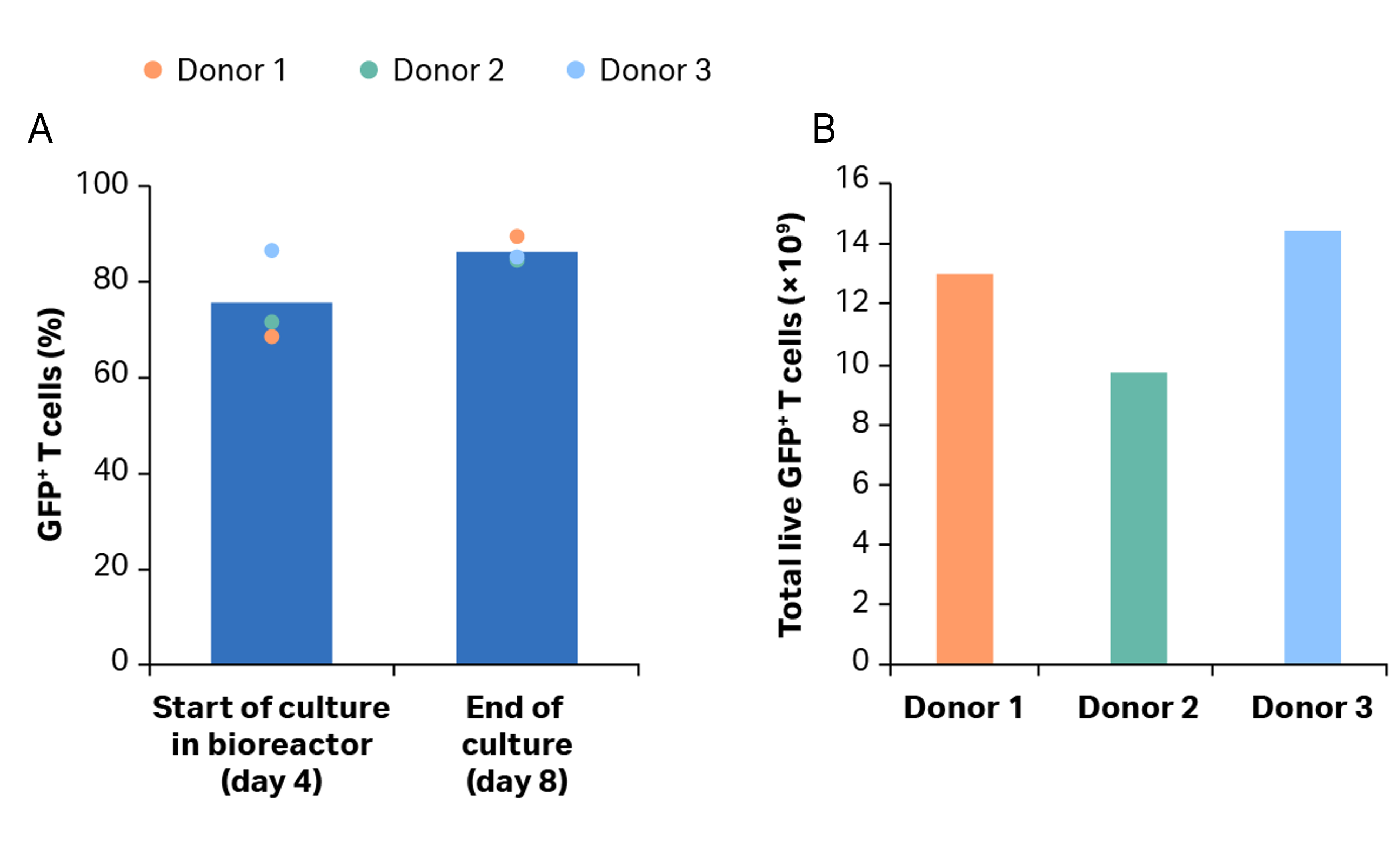
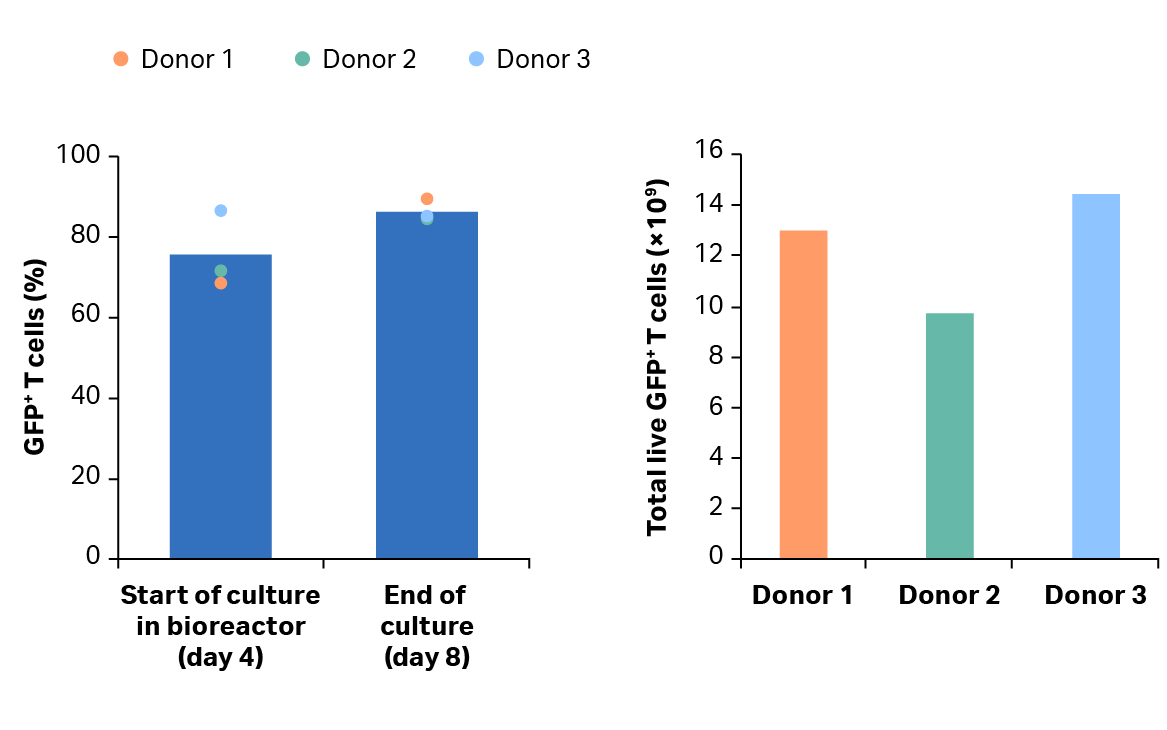
Fig 6. Transduction efficiency. (A) percentage of transduced GFP+ T cells after activation in shake flasks and after culture in the Xuri Cell Expansion System W25. Bars represent the average of the biological triplicates with individual donor values shown by colored dots; (B) cumulative GFP+ T cells on day 8 for donors 1, 2, and 3.
Final steps in CAR T cell manufacturing workflow
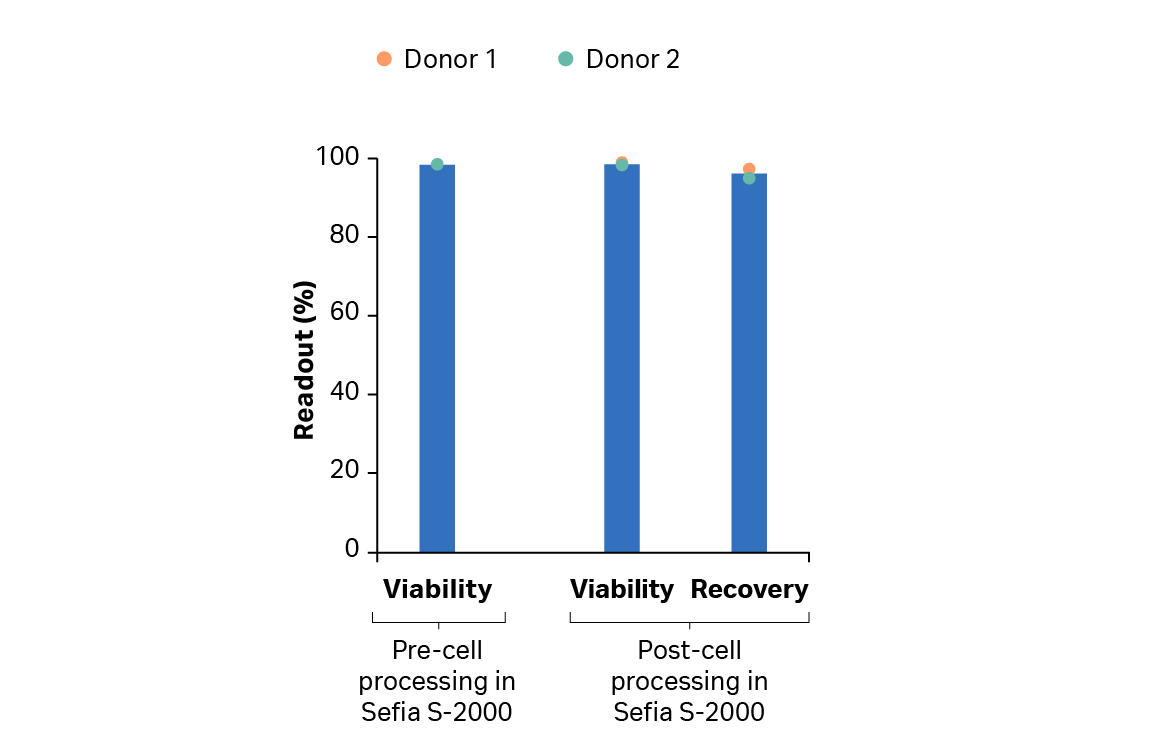
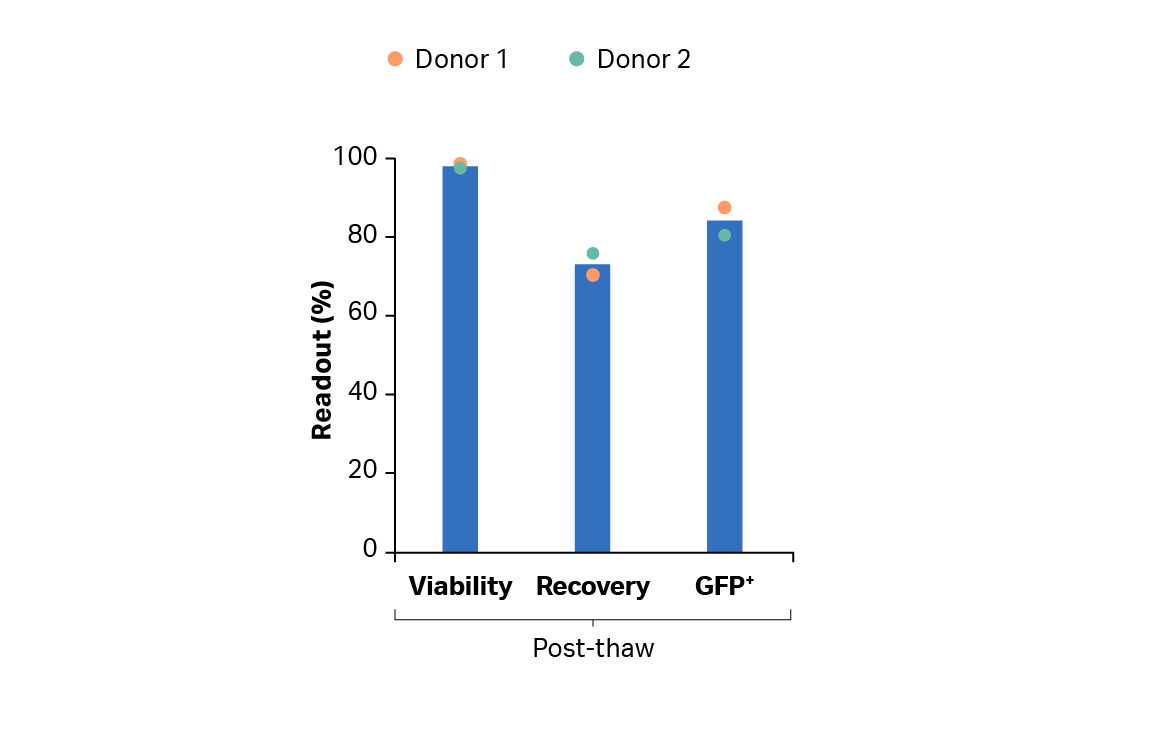
After the expansion step, we used the Sefia system for T cell harvesting, washing, and formulation. Recovery was > 90% (Fig 7). The VIA Freeze system performed controlled-rate cryopreservation with > 70% recovery and > 97% viability post-thaw. The results also show that transduction was maintained above 80% throughout the cryopreservation process (Fig 8).
Fig 7. Recovery and viability of cultured T cells from donor 1 and 2 after harvest using FlexCell Protocol Software on the Sefia S-2000 instrument.
Fig 8. Post-thaw recovery and viability of transduced T cells cryopreserved after cryoformulation using FlexCell Protocol Software on the Sefia S-2000 instrument.
Collectively the studies described here, along with additional analyses, informed development of a semi-automated and closed CAR T cell manufacturing process (Fig 1).
Summary: workflow for commercial CAR T cell production
Here we outline development of an advanced CAR T cell production process. Manual, open, and disconnected unit operations have been substituted for options that support automated, closed, and integrated processing.
This study shows:
- Robust closed processing and automation strategies to manufacture clinically relevant doses of CAR T cells.
- A flexible end-to-end workflow that is suitable for industrialized autologous CAR T cell manufacturing and can be adapted for GMP compliance.
Read the full article on development of a robust CAR T cell process.
Learn more about process development for cell therapies.