FDA’s current good manufacturing practice (cGMP) regulations are a critical factor in producing safe and effective biologics. When a manufacturer complies with cGMP regulations, the process, facilities, equipment, and controls used to make the product will consistently produce a quality drug for human use. The regulations, 21 CFR Part 210 and 211, as well as the applicable requirements in 600 through 680 and 820, outline the minimum requirements for biopharmaceutical manufacturing. These include controls over personnel, facilities, equipment, components, processes, testing, packaging, labeling, warehouse, and distribution. Traceability of these controls is a key aspect of ensuring compliance to all cGMP requirements.
As the industry continues to grow and evolve, advanced therapies are now being produced in unique settings. These settings include hospitals, translational facilities, and facilities for small-scale manufacturing. Advanced therapies are also being developed using new processes, such as the genetic modification of a patient’s own cells. This creates major challenges in cGMP compliance, because these locations and processes might not be equipped to monitor and safeguard production. Even biomanufacturers with cGMP facilities designed for biologic production face obstacles when trying to ensure the stability, progression, and traceability of autologous cell therapies from the time the cell material reaches their facility to when it leaves. Simplifying the day-to-day tasks of managing these cutting-edge drugs and ensuring they are consistently produced is vital to the future of the industry. Therefore, as automation continues to offer new options for flexibility and optimized production in biologic production, exploring its use in advanced therapy manufacturing to improve safety, quality, and compliance becomes a key factor in advancing patient care.
Cell therapy manufacturing: today and tomorrow
Biomanufacturing has always presented unique challenges because it uses living organisms to develop medicines. The changing nature of these organisms makes it much harder to guarantee consistency than when producing drugs through chemical reactions. These obstacles are magnified in cell therapy manufacturing, where sterility assurance is a key process attribute because the final product cannot be sterilized. Human intervention, either through open manipulation steps or removing in-process samples, creates variability and risks the sterility of the cell therapies. Thus, manual processes open up the possibilities of contamination and inconsistent therapies.
Also, autologous cell therapies require traceability during all steps of the process to ensure the patient’s own cells are delivered back. A major consideration for the future of cGMP-compliant manufacturing of these therapies is to combine automated, closed system manufacturing technology with electronic systems for record keeping, process controls, and audit trails. Closed systems and automated processes provide a higher level of contamination control and manufacturing consistency. Further, electronic record systems provide the documentation for traceability and system performance.
Today, many manual steps in cell therapy manufacturing are time dependent. For example, typical workflows require waiting an average number of days for cell proliferation and population doubling based on previous development work. This wait time is employed because collecting samples for analysis on every batch at multiple points could compromise sterility. The ability to monitor the entire manufacturing process within a closed system allows staff to track operations. It also lets them look at the health of the cells and the consistency of the process in real time, so they can identify and control any trends that could impact the final product. Better monitoring of these parameters will help to replicate a high-quality manufacturing process. Ultimately, if an automated system can provide an aseptic barrier and the critical process parameters are maintained throughout the process, this could also lead to significant time and cost savings.
While manufacturing execution systems (MES) that offer these capabilities are used in bioprocessing today, they often come at a very high price tag. This cost might be too high when producing therapies at a small scale or during clinical trial development. Also, bioprocessing MES are not specifically designed for cell therapy manufacturing, where tracking the chain of custody of a cell line is essential to proper handling of personalized medicines. As a supplier dedicated to providing advanced solutions and application support for biopharma, we recently launched Chronicle automation software. This is the first cell therapy manufacturing solution developed by cell therapy platform engineers. Chronicle is designed to help optimize complex cell therapy processes while demonstrating regulatory compliance and creating a pathway for rapid adoption. Understanding what the software does and the benefits it offers might be the key to implementing an automated system in your facility. Such a system would simplify your complex processes and ensure that your process delivers consistent and compliant treatments for the patients you serve.
Chronicle automation software for cell therapy
Developing and manufacturing cell therapies is a vital task that requires profound care and efficiency. If something should happen to patients’ cell material during manufacturing, the responsibility falls on the team responsible for the safe handling of these potentially life-saving therapies. Putting in place a system to safeguard these processes could lead to lower costs, increased throughput, expert staff who feel supported in their roles, and a greater possibility of meeting the patient dose requirements.
Mitigate risk through alarms, monitoring, and SOPs
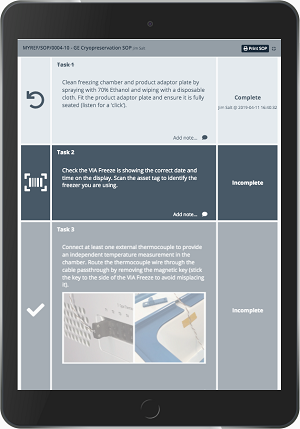
Chronicle users can benefit from a unified digital space by monitoring various elements in manufacturing and across their supply chain with real-time data acquisition and alarms supported by standard operating procedures (SOPs). Because Chronicle is a software-based web application optimized for smartphones and tablets, you can, for example, receive an alert via text or email notifying you that something is wrong. Then, you can respond right away through the system to address the issue. Creating electronic SOPs (eSOPs; Fig 1) allows you to use digital tools to track your manufacturing operations across batches.
Fig 1. Example of an electronic standard operating procedure (eSOP) from Chronicle software.
Collect and store true automated batch records
Chronicle also offers efficiency through automated batch records. A batch record for a therapy can run hundreds of pages long. These pages often must be drafted by hand, reviewed, and matched to achieve traceability. Instead, Chronicle captures various elements in manufacturing, such as executed process steps and process deviations. The software also captures product information, including lot ID and expiration dates. It automatically scans QR codes on consumables (Fig 2), samples, and instruments, including detailed data from connected equipment. Then, Chronicle stores all the data in a secure cloud with unlimited storage. These electronic batch records can be collected, saved, and archived for later access, ensuring traceability for years to meet regulatory requirements.
Fig 2. An operator scans a reagent label to identify lot number and expiration date.
Get up and running quickly
Chronicle is a GAMP™ 5 Category 3 software, which means it was developed to meet the specific needs of the regulated company (1). It requires only days or even hours to install, rather than months. Further, Chronicle can connect to and integrate with third-party equipment, and it can be accessed across multiple roles in a manufacturing setting. For example, a quality team can look at how many deviations have occurred and whether training requirements for the people using the equipment have been met; supply chain experts can monitor the number of consumables used in a period of time, their expiration date, and how they are being disposed; and a logistics team can check the number of shipments in the queue and how many have already occurred.
Also, Chronicle software is delivered as a software as a service and pay-per-use model that is based on how many batches you plan to process, whether it is at a commercial scale or just one batch in a small-scale setting. For example, Vanderbilt-Ingram Cancer Center recently partnered with us to use automation and digitized workflows to improve productivity in its cell processing operations, including using Chronicle software. A system that can grow with you and your needs offers flexibility that is critical for a market expanding as quickly as cell therapy.
Scale as you and the industry grow
Chronicle works for both non-cGMP and cGMP environments. This offers the option to start small with an accessible, flexible solution for investigational or development work. Then, when the time is right, it lets you scale-up and lockdown processes to manage efficient, consistent production with the potential for thousands of connected units across multiple continents. The ability to maintain consistency in your operations at multiple scales is important not just to a drug manufacturer but also to the industry. This is true because the technology we use to advance our manufacturing processes to meet cGMP guidelines must be able to evolve and advance with the science used to create life-saving medicines. For instance, new vaccine manufacturing evolved from using egg-based manufacturing to much more consistent and efficient cell culture production. Cell therapy manufacturing is also evolving as new manufacturing systems and techniques are developed. Using a patient’s cells to prevent, diagnose, and treat disease has led to exciting new opportunities in treating cancer and a wide variety of rare diseases. Without advancing our manufacturing technology to enable the safe and consistent production of new therapies, we risk stalling the advances in science that are already bringing new hope to so many patients in need.
Read more about Chronicle manufacturing automation software.
References
- AmpleLogic i-QMS. Brief on GAMP 5 Categories, V Model and 21 CFR Part 11, EU Annex 11 (2018). [Online.] https://www.amplelogic.com/blogroll/brief-on-gamp-5-categories-v-model-and-21-cfr-part-11-eu-annex-11/. Accessed 29 October 2019.