His-tagged protein purification is popular and commonly used in research labs. Here are 3 tips to ensure that you get the most from your his-tagged protein purification protocol.
Tags simplify purification and enable scientists to use standard protocols. It improves the yield, purity, and solubility of the protein of interest, and minimizes the number of purification steps needed. We have gathered tips for purifying his-tagged proteins.
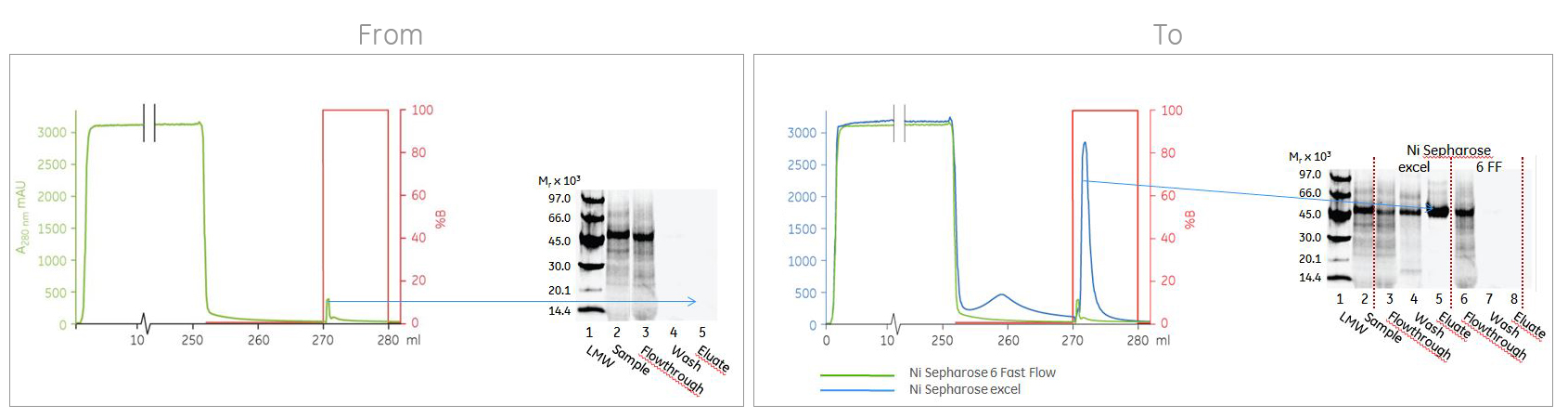
1. Low or no recovery of your his-tagged protein? Change to an EDTA-resistant resin.
Your cell culture medium could contain components that mess up your yield. Immobilized metal affinity chromatography (IMAC) purification of his-tagged proteins secreted into eukaryotic cell culture supernatants has traditionally been challenging, because cell culture media are often incompatible with traditional IMAC resins. The cell culture medium can strip the immobilized metal ions from the IMAC resin during sample loading. As a result, the target protein might bind poorly or not at all. By switching to an EDTA-resistant resin like Ni Sepharose excel, that problem will be long gone!
Also, check out our blog post on low yield of his-tagged proteins.
2. Need to remove the his-tag? Use on-column cleavage.
One of the benefits of using the his-tag is its small size, which means that often it can be left on the protein. But sometimes the his-tag can interfere with your protein’s function, or you might need your protein in a native state. To simplify the cleavage step, you can add a his-tagged protease, like his-tagged TEV or HRV3C, while your tagged protein is still bound to the IMAC resin. And voilà – the flowthrough will contain the cleaved protein of interest. The his-part of the tagged protein and the protease itself will remain bound to the IMAC resin.
See our easy protocols for removing the tag and protease, whether your protease is tagged or native.
3. Want to improve your results? Go automated!
Purifying proteins manually allows for easy set-up and does not require instrumentation such as pumps and valves. However, there are a few drawbacks to manual protein purification, such as:
- Potential chromatography resin loss when removing supernatant
- Process is time consuming and labor intensive
- Potential protein loss due to many steps
With an affordable and easy-to-use protein purification chromatography system such as the ÄKTA start, you will get:
- Better reproducibility than with manual purification
- On-line monitoring – you can easily see which fractions to pool
- Time and labor savings compared with manual purification
Read more about how to avoid the hassles of manual purification.