Your challenge
Scientists use cryo-electron microscopy (cryo-EM) to perform structural studies on large multi-subunit protein complexes. Cryo-EM technology has evolved along with the increasing need for small-scale preparative purification of these complexes.
That’s because cryo-EM can help scientists work with lower protein amounts of large complexes from insect, mammalian recombinant, or even native expression systems. Theses complexes tend to be rather unstable, which can make their purification challenging.
When working with recombinant expression systems, researchers use affinity chromatography for capture. They’ll often add an intermediate polishing step ion exchange chromatography due to the high-purity level needed. For the best results, the sample should be monodisperse in solution, enabling size-exclusion chromatography (SEC) to be used in the final polishing step in a purification protocol.
Once purified, scientists apply the sample to the cryo-EM grid in small drops of about 4 µL in concentrations from 0.1 – 5.0 mg/mL. To keep the sample monodisperse, researchers try to avoid additional concentration steps after the final SEC polishing step. When working with small-scale preparative columns, such as Superdex™Increase 3.2/300, when yielding peaks of approximately 50uL, a chromatography system which can maintain resolution to the point of fractionation is required.
Introducing ÄKTA pure™ micro
Cytiva is helping improve cryo-EM processes with ÄKTA pure™ micro system.
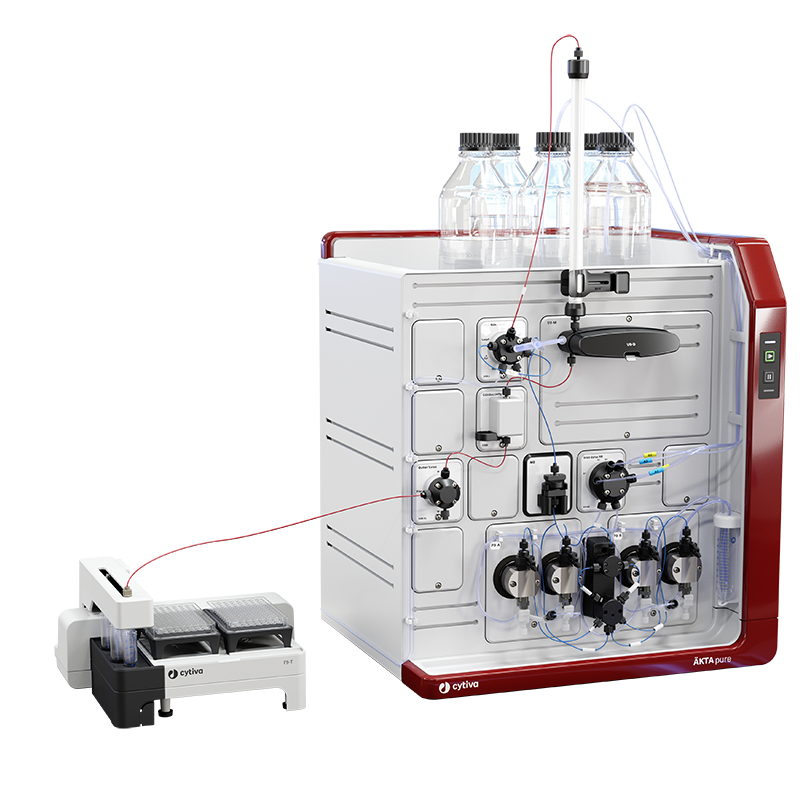
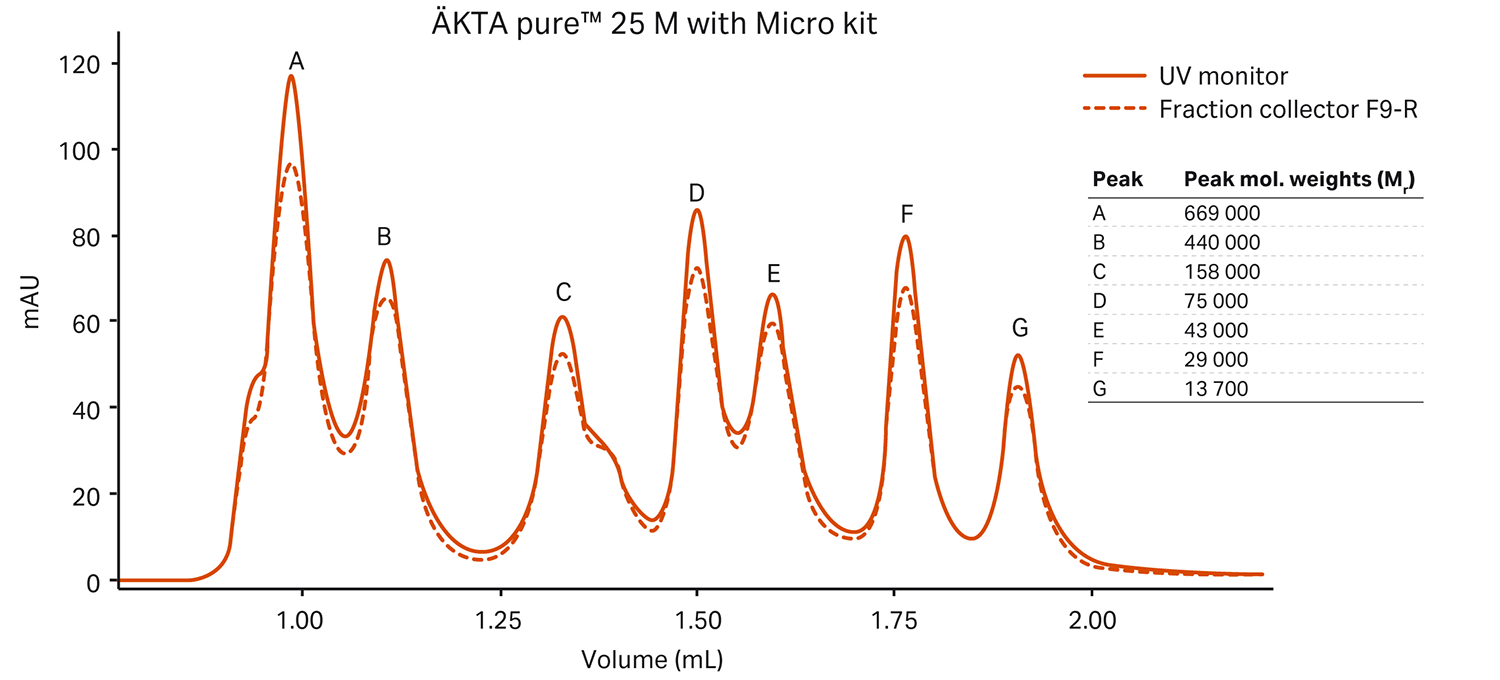
The ÄKTA pure™ micro system has a flow path optimized for high-performance microscale purification. It’s designed to minimize peak broadening between the UV monitor and the fraction collector, as well as between the injection valve and the column. Combined with the fraction collector F9-T and micronozzle, it helps you successfully purify small sample volumes before collection without diluting your peaks. You can use the micronozzle to collect small fractions around 40 µL.
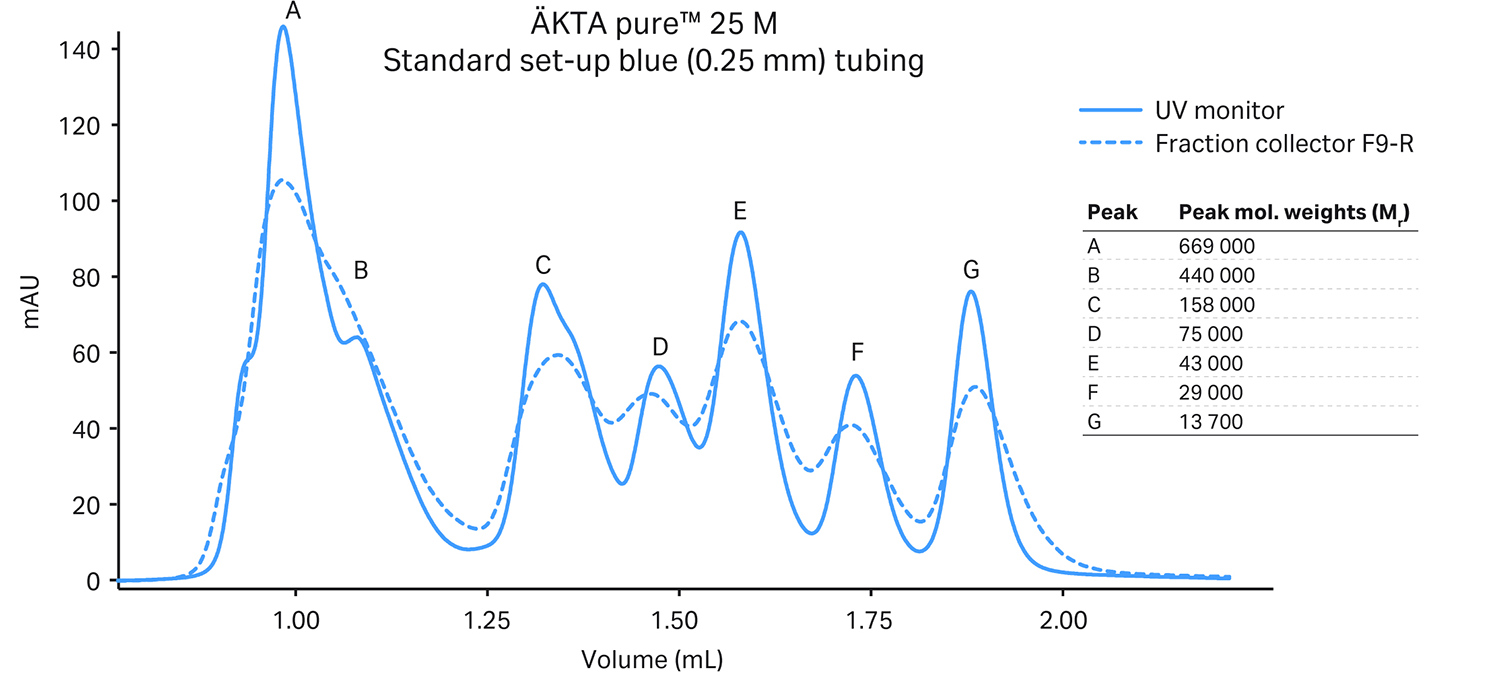
The figure below shows how our ÄKTA pure™ micro system helped maintain the resolution of a Superdex™ 200 Increase 3.2/300 column (18 µL delay volume UV monitor to fraction collector). We compared the results to an SEC-optimized standard ÄKTA pure™ 25 system (50 µL delay volume):
In addition to the preconfigured ÄKTA pure™ micro system, we also offer a Micro Kit you can use to convert your ÄKTA pure™ 25 M to an ÄKTA pure™ micro system.
Comparing ÄKTA pure™ micro to ÄKTA™ micro system
Professor Hopfner’s group at the Gene Center of the LMU Munich is investigating how cells shape and protect their genetic information. For them, cryo-EM is an essential tool to study the architecture and functions of macromolecular complexes. The team uses the classic ÄKTA™ micro system to purify samples.
Dr. Gregor Witte and Stephan Woike from Professor Hopfner’s team tested ÄKTA pure™ micro and compared its performance to the classic ÄKTA™ micro system in their lab. In their own words, here is what they think of the new system.
Test case: In vitro reconstitution of a DNA-binding trimeric protein complex
As a structural biology group, we use a wide range of protein chromatography methods on a daily basis. As scientists apply cryo-EM more, there’s an increasing need for small-scale purification of larger and dynamic protein complexes. We’ve been using a classic ÄKTA™ micro system for IEC and SEC, particularly for the last purification step before EM grid preparation.
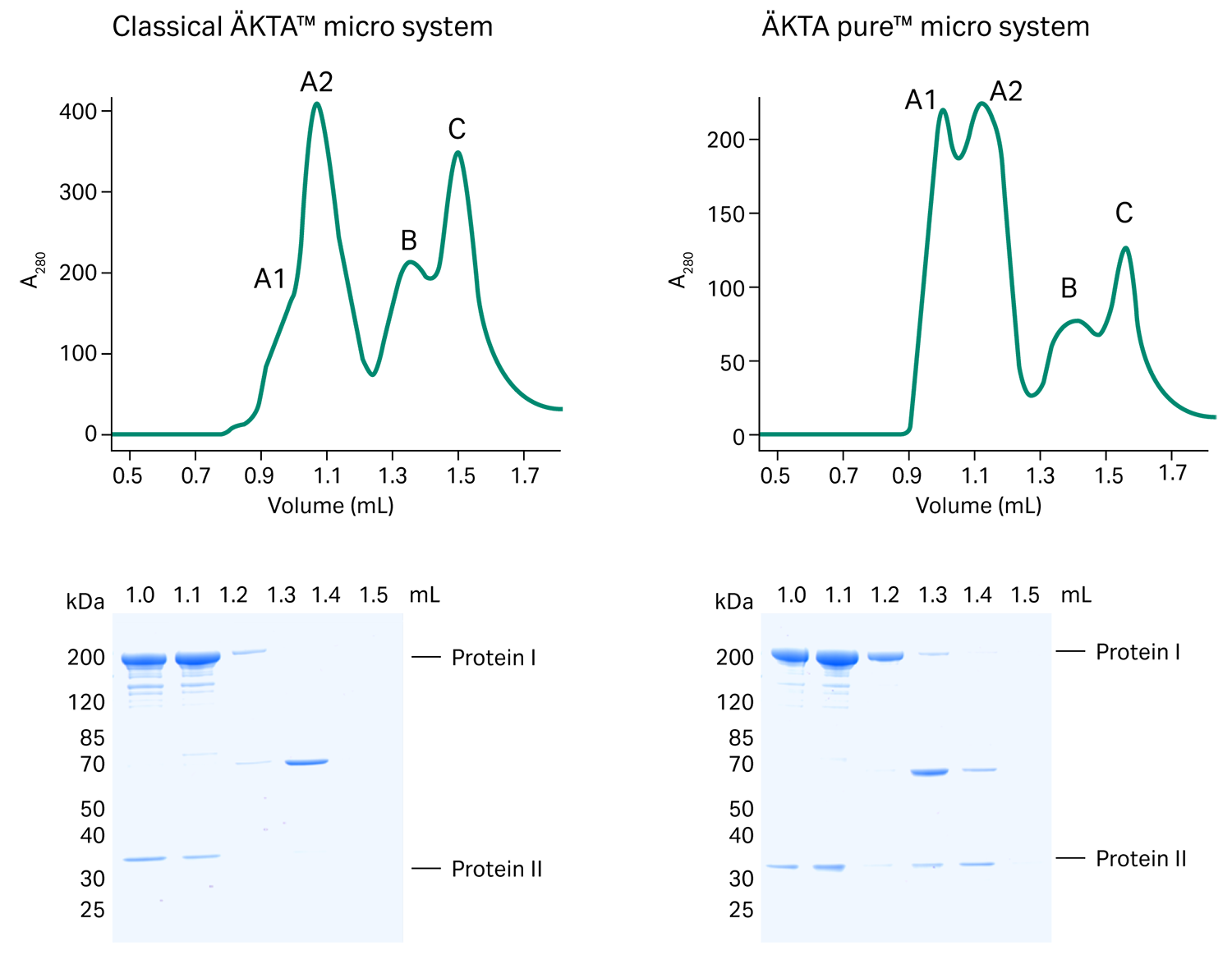
To compare how ÄKTA pure™ micro performs against the classic ÄKTA™ micro system, we purified a DNA-binding trimeric protein complex on a Superdex™ 200 Increase 3.2/300 column injected through a 30 µL loop.
We had reconstituted the complex in vitro by consecutively adding Protein I (180 kDa) and Protein II (32 kDa) to DNA. Besides some minor differences, the main absorption peaks eluted at similar retention volumes. Both ÄKTA™ systems showed excellent separation of the target complex (peaks A2) from protein impurities (peak B) and excess DNA (peak C). The target complex tended to homodimerize (peak A1). A dynamic equilibrium between monomer and dimer formation explains the differences in the height of peak A1.
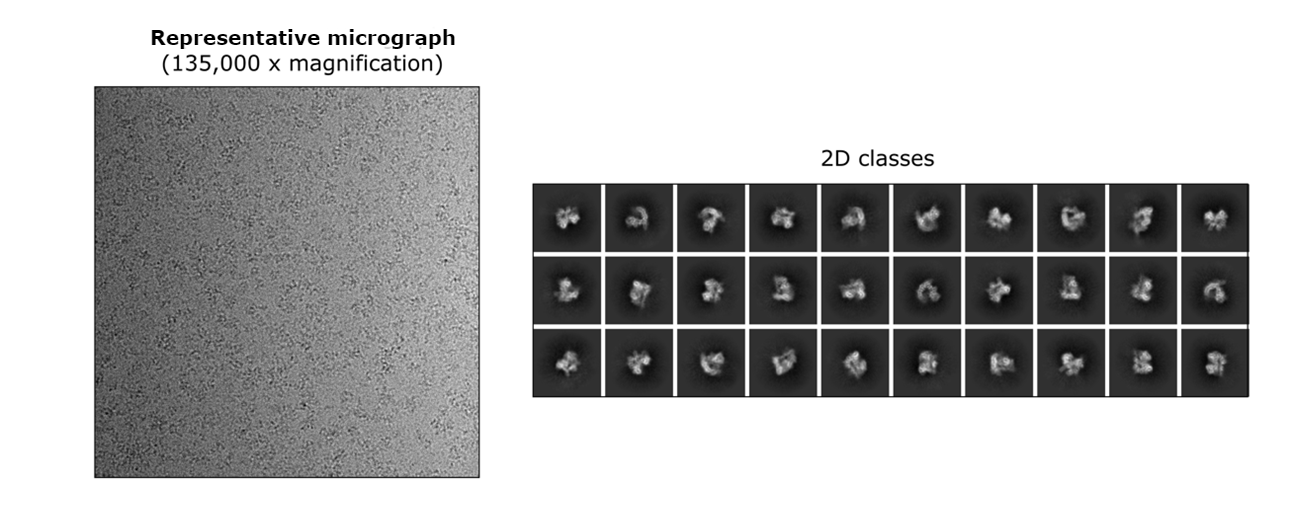
EM grid preparations of the target fraction (dashed square) represented by peak A1 led to a homogenous sample distribution on micrographs and detailed 2D classes. The ÄKTA™ micro system was an indispensable part of our setup that helped us resolve four different conformations of this DNA-binding complex using cryo-EM.
It was very straightforward for us to handle the new ÄKTA pure™ micro, and the process was almost identical to the one we used with ÄKTA pure™ system. We could flexibly control the fraction collector F9-T using UNICORN™ software — which enabled us to use one well plate for multiple chromatography runs. Also, we could use different plate formats thanks to the fraction collector’s versatility. In our experience, we were even able to reliably fractionate volumes smaller than 50 µL, which could help improve other applications in small-scale structural biology.