Viral clearance studies are a critical part of the production of biologics. However, performing studies that are accurate and cost-effective can be challenging. Here are seven things to consider while performing your next chromatography viral clearance study.
- Select a suitable scale-down model
- Use a column with a narrow inner diameter
- Know when to choose prepacked or empty columns
- Don’t compare compression factors
- Challenge your chromatography column
- Perform an integrity test on the column
- Use manufacturing feedstock
1. Select a suitable scale-down model
For chromatography steps, the scale-down model should closely mimic the large-scale step to represent what occurs in the manufacturing process, while limiting the amount of sample and resin used. Critical parameters to consider when selecting a suitable scale-down model include protein load to column volume ratio, flow rate to bed size, as well as buffers, pH, yield, and purity.
Remember to always validate the small-scale column before the viral clearance study to ensure it truly represents the full-scale process.
2. Use a column with a narrow inner diameter
The bed height and flow rate used in the scale-down model should be the same as in the full-scale step. The main difference is that the inner diameter of the column is narrower. It is common to use a scale-down column with a diameter of 10 mm as this size is a good representation of a large-scale step but saves both resin and sample.
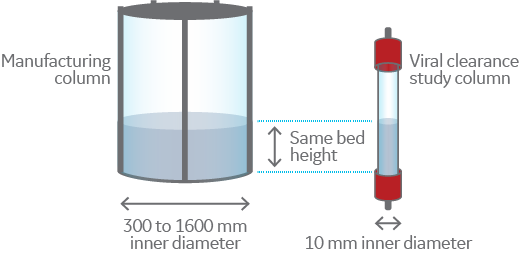
You should avoid using a column with an inner diameter that is too narrow as it might not accurately represent your large-scale process due to the supporting effects of the column wall. As the column diameter decreases, the supporting wall effects on the bed stability increase so it’s important to select the proper size column for your study.
3. Know when to choose prepacked or empty columns
You need several chromatography columns for each virus clearance study so it’s essential to carefully consider which columns you select for your study.
You can either use a prepacked column or pack an empty column with a suitable resin yourself. In either case, it is essential to obtain a high-quality packed resin bed to ensure reproducible results between runs and reduce the risk of failure during the study.
The table below summarizes the pros and cons of these two options.
| Empty columns to be packed with chromatography resin | Prepacked columns already packed by the column supplier | |
| + |
|
|
| - |
|
|
4. Don’t compare compression factors
The compression factor (CF) increases with column size. For that reason, you can’t compare the CF for the small-scale column with the CF for the full-scale column.
5. Challenge your chromatography column
Before the study, test the small-scale column with high flow rates of more than 500 cm/h. That way, if the high flow rates create a gap in the packed bed, you have an opportunity to repack your column without it impacting your study. It’s better to discover issues like these before, rather than during the actual study.
6. Perform an integrity test on the column
Even well-performed viral clearance studies require a substantial number of columns over a year’s time. To avoid incurring additional costs, it’s important to ensure the bed is packed correctly.
To minimize this risk, you should always perform an integrity test on each column first. The integrity test usually includes a check of asymmetry factor and efficiency. And if the columns pass the test, you know it is of high quality and packed well before moving into viral clearance.
7. Use manufacturing feedstock
When you are performing the viral clearance study for the chromatography step, make sure to use feedstock/cell culture harvest from the production process batch. This will prevent variation in impurity profiles, which could affect the viral clearance log reduction of the step.
If you want to learn more, read this case study describing a recent viral clearance study of an affinity step.