Cancers. Genetic mutations. Rare diseases. Discovering new ways to detect and treat these conditions and other previously untreatable diseases has motivated the recent growth in the field of oligonucleotides, or oligos. These strands (10 to 200 nucleotides in length) of synthetically made DNA and/or RNA hold the potential to revolutionize current treatments. And the variety of applications continue to spread at an astonishing pace.
Nucleic acid therapeutics include diverse types of nucleotide chains notably DNA plasmids (pDNA), mRNA, and oligonucleotides. Each nucleotide type differs by molecular structure, therapeutic indication, and manufacturing process. From a manufacturing perspective, pDNA and mRNA are produced by biological processes, while oligonucleotides are synthetically synthesized and are commonly produced using flow-through, solid-phase synthesis. Synthetically made oligonucleotide therapeutics and diagnostics are commonly classified as short oligos (10 to 50 nucleotide bases long) or long oligos (50 to 200 bases long).
Clustered regularly interspaced short palindromic repeats (CRISPR) technology is a gene-editing technique breaking new ground. The first drug using this technology has recently been approved. The drug design is dependent on a long guide RNA (gRNA) sequence that is produced using this very same synthesis technology, pushing the technology to its limits for quality outcome.
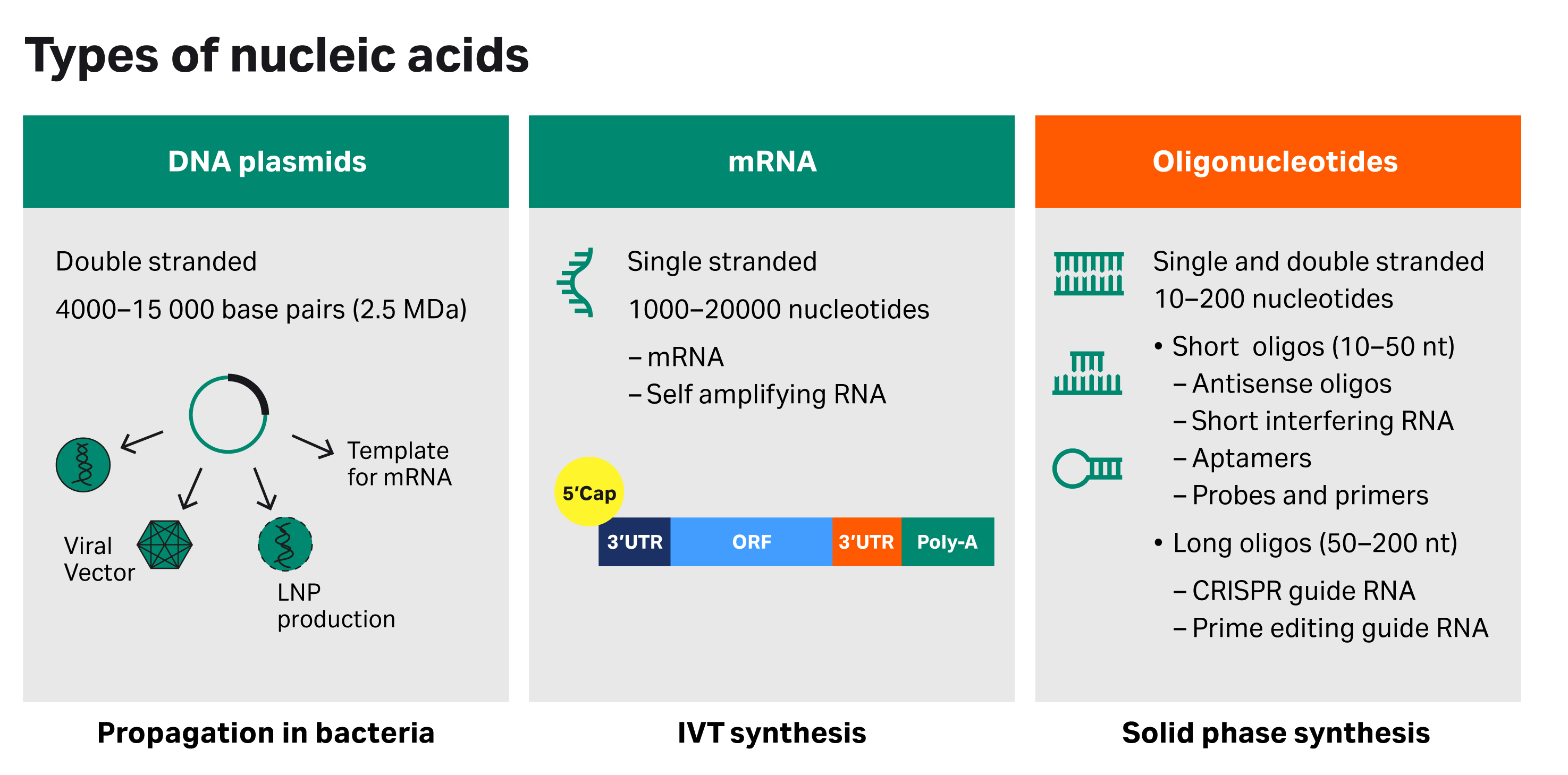
Fig 1. Types of nucleic acids
The short oligo market
Short oligos, such as antisense oligos (ASOs) and short interfering RNA (siRNA), lead the way in both preclinical and commercial markets. Their efficacy, especially for previously untreated conditions, has built the foundation for personalized genomic medicines. (see Papasavva et al. [2], Table 1). These ASO and siRNA sequences are made up of similarly modified nucleotides, but generally ASOs are single stranded, and siRNAs are double stranded. Despite their differences, both work by targeting mRNA in the cell to alter protein expression and benefit the patient through a variety of mechanisms.
Looking to the future in oligo therapeutics, ASOs and siRNA therapeutics will continue to lead the clinical pipeline and FDA approvals as shown in Figure 2. Of the approximately 1500 oligonucleotide therapies in the clinical pipeline, more than 60% are either ASOs or siRNA oligonucleotides. As of December 2023, there were 18 FDA approved oligo therapies, with the majority being either ASOs (10 approved) or siRNAs (five approved) (1).
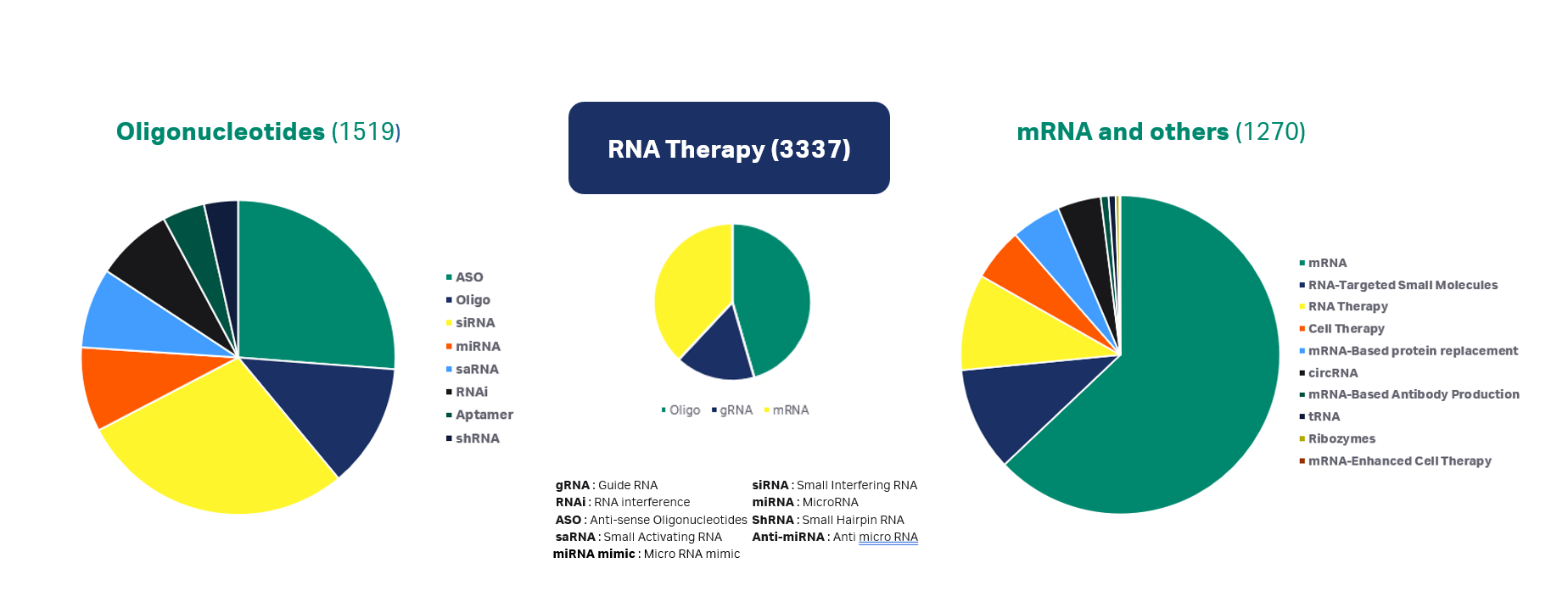
Fig 2. Oligonucleotides and mRNA therapies in the clinical pipeline comparison
CRISPR and long oligos
While ASOs and siRNAs hold the market majority of FDA approved therapies, long oligos are poised for explosive growth thanks to CRISPR technology. CRISPR is a gene editing technology derived from the bacterial adaptive immune system that can revise, remove, and replace genes in a highly accurate manner. The technology uses a Cas protein, so the overall technology is often called CRISPR/Cas or CRISPR-Cas. It also requires a long-oligo gRNA sequence produced from synthetic RNA bases. This technology was realized in 2012 (3) and resulted in Jennifer Doudna and Emmanuelle Charpentier winning the Nobel Prize in Chemistry in 2020.
The secondary structure of long oligo sequences is essential for the CRISPR/Cas system to function, as illustrated in in Figure 3. CRISPR gene-editing technology allows genomic DNA to be actively manipulated in the laboratory and is delivering incredible results in the clinic across numerous applications. With the revolution of CRISPR gene-editing technology, the need for long gRNA oligo production is increasing.
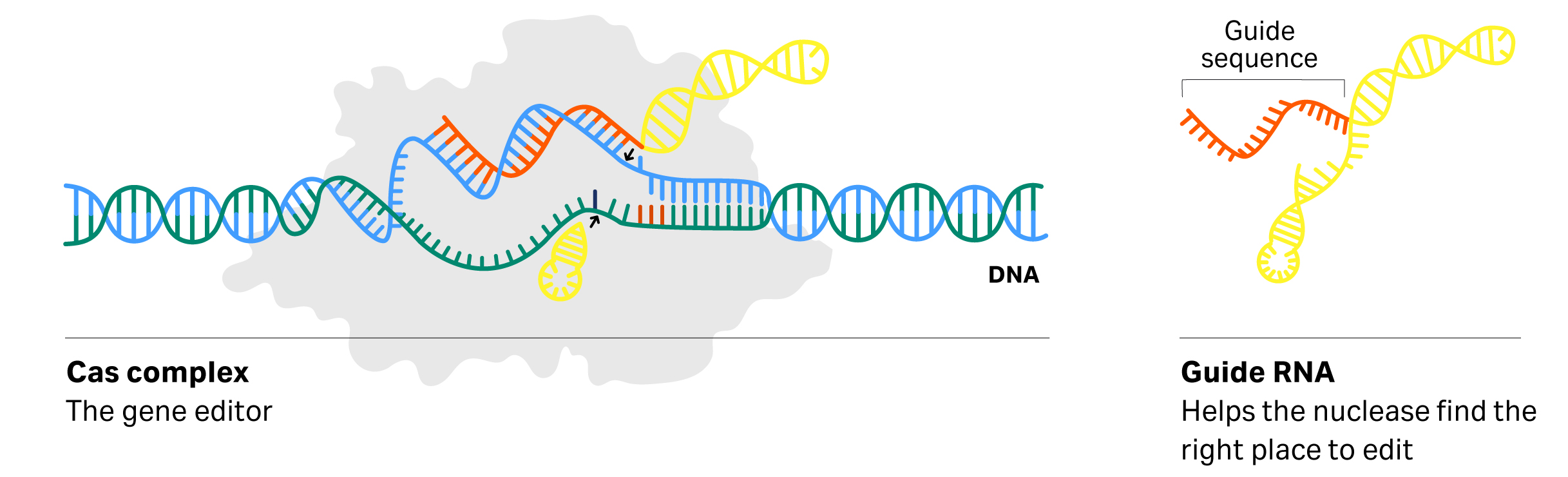
Fig 3. Secondary structure of gRNA and the Cas nuclease
CRISPR/Cas editing is commonly referred to as genomic scissors because it can alter the genome by cutting out sections of DNA that cause a certain disease, commonly referred to as knock-out edits. It can also be used to knock-out a section of DNA and replace it with a different sequence for a knock in edit.
In the CRISPR/Cas complex, the guide RNA acts as the scissors, while the Cas protein can be thought of as the hand that uses the scissors to cut the DNA. While the Nobel Prize research was done using CRISPR/Cas9, there are now many different types of proteins (the hand) that require different types of guide RNA (scissors), an example is Cas12a shown in Figure 4.
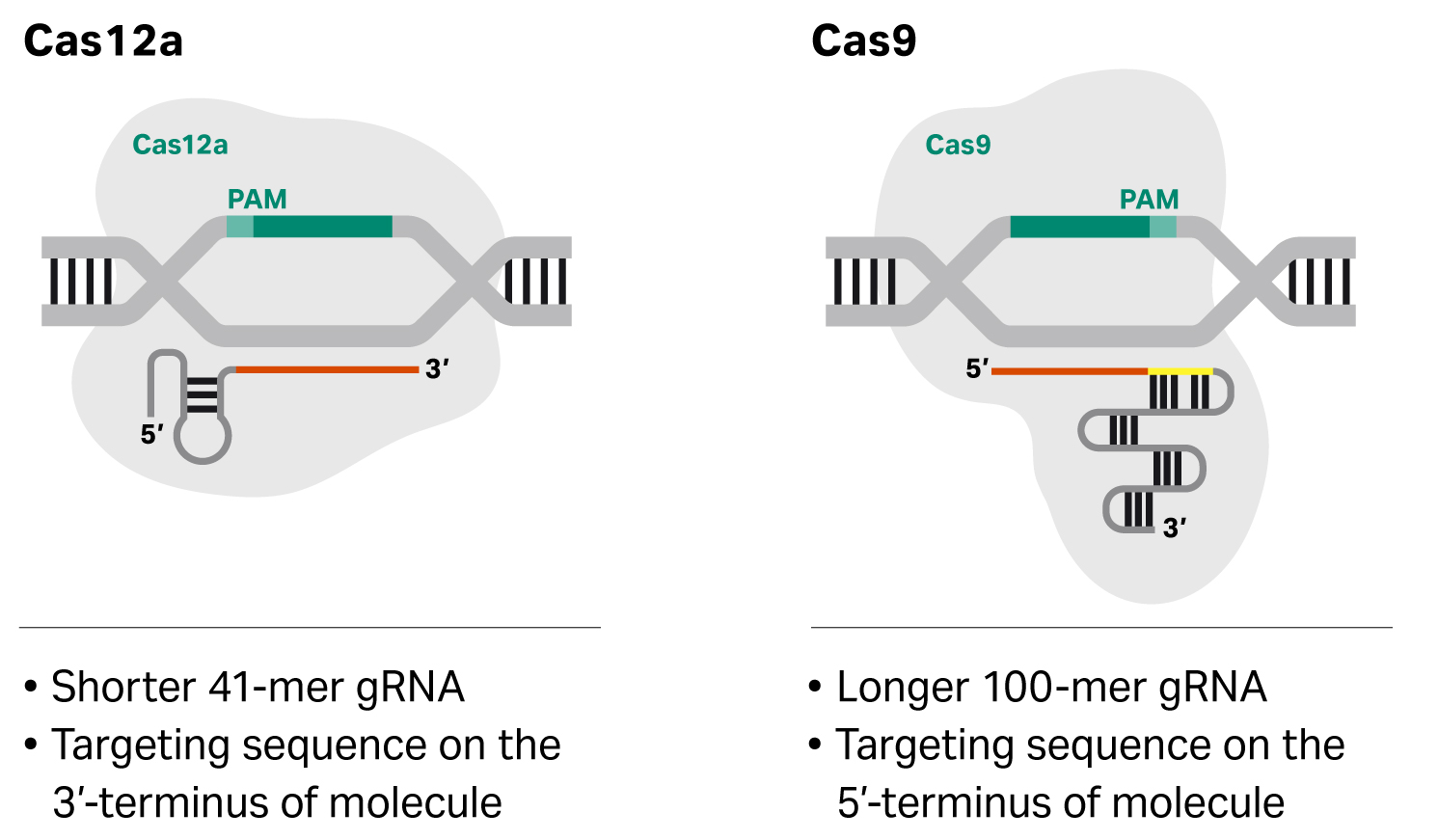
Fig 4. Cas9 and Cas12a require different gRNA (4)
The Cas9 and Cas12a CRISPR examples are just two of the many different types of gene editing that are currently being developed. There have been constant breakthroughs with new types of Cas proteins as well as an entirely different categories of gene editing.
The long oligo market
CRISPR/Cas technology has led to an explosion in the long oligo market. For example, Tome Biosciences recently announced that it is developing technologies designed to insert large amounts of genetic material anywhere in the genome without damaging or breaking DNA (5). CRISPR/Cas in vivo, ex vivo, and edited cell-based therapies are expected to continue double-digit growth and will likely surpass siRNA in 2029. CRISPR technology already contributes to 15% of the market (1). This gene editing industry is predicted to be worth around $36 billion by 2027 and will continue to grow (6).
The rapid expansion of this technology has proportionally grown the demand for long, synthetic gRNA and the need to overcome significant challenges in manufacturing and analyzing these complex molecules.
Considerations for synthesis of synthetic long oligonucleotides
The synthesis of guide RNA sequences does not come without consideration. The synthesis of gRNA is commonly relegated to small scales (< 1 g/batch) due to the low crude yield and purity of gRNA because of their length (Fig 5) and the challenging coupling efficiency of 2′-O-tert-butyldimethylsilyl (2’TBDMS) RNA bases. The synthesis challenges are further complicated by the use of low-loaded controlled pore glass (CPG).
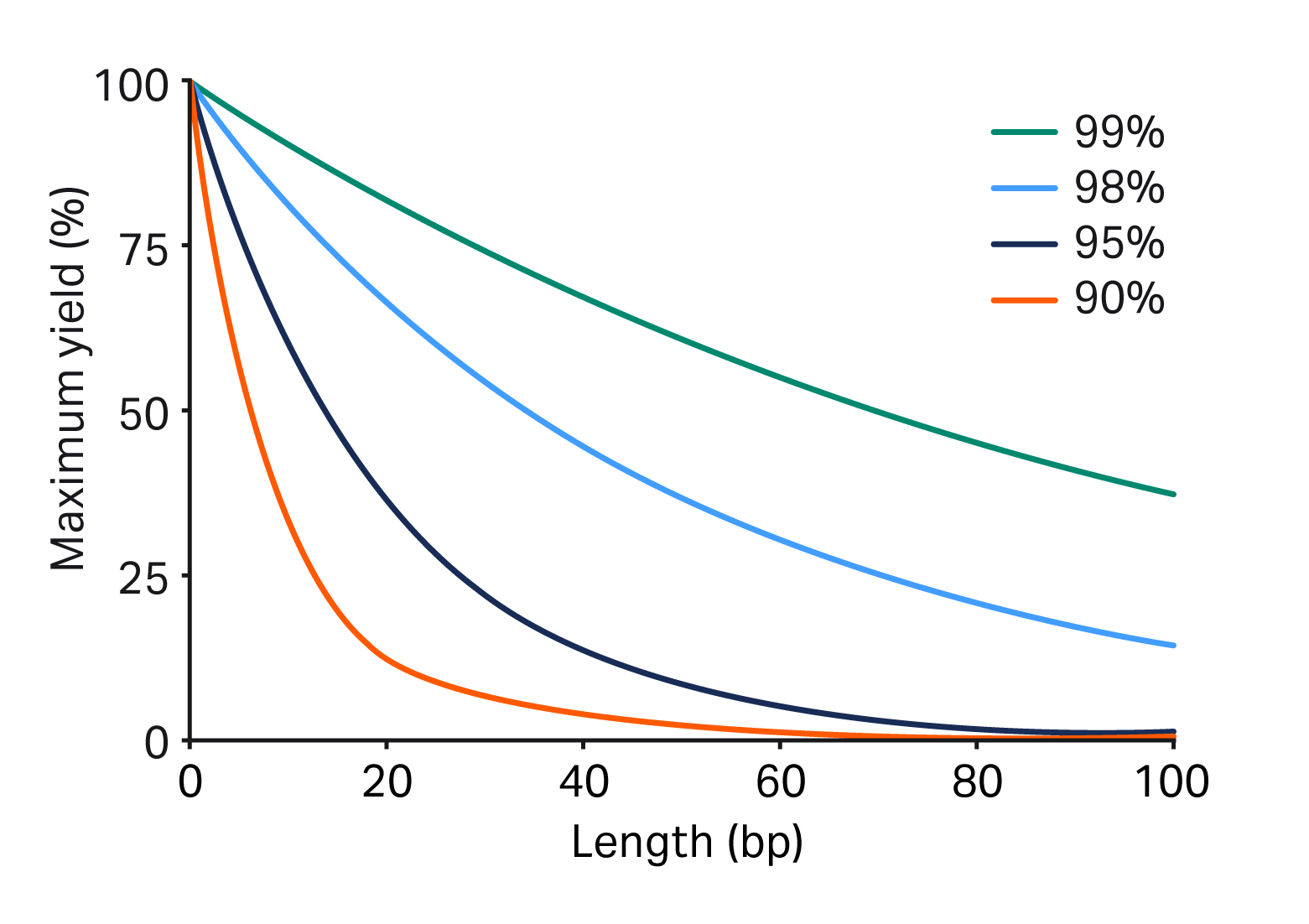
Fig 5. Decreasing yield and purity with oligo length varies with the coupling efficiency of 2’TBDMS
In addition to poor coupling efficiency, 2’TBDMS RNA commonly requires a special chemical deprotection with hydrofluoric acid. Hydrofluoric acid requires specialized safety and vessel compatibility considerations. The manufacturing challenges of gRNAs continue for their purification. One must overcome the residual hazardous organic solvents from the deprotection process, secondary structure interactions, their size (> 30 kDa), and the relatively low crude purity (30%) during the purification of these molecules.
Conclusion
In summary, the siRNA, ASO, and gRNA market shows exciting growth and will need new developments to make the manufacturing process more efficient and sustainable. Cytiva continues to navigate the changes and innovations in oligonucleotides and genetic editing. Our history in the oligo field, our portfolio of reliable solutions, and our unparalleled application and field service will help you reach your oligonucleotide goals, no matter the length or target scale. We are dedicated to supporting our customers with exceptional equipment, service, and application support for all oligos long and short.
- Beacon data last accessed October 2023 https://data.beacon-intelligence.com/
- Papasavva P, Kleanthous M, Lederer CW. Rare opportunities: CRISPR/CAS-based therapy development for Rare Genetic Diseases. Mol Diagn Ther. 2019;23(2):201-222. doi:10.1007/s40291-019-00392-3
- Jinek M, East A, Cheng A, Lin S, Ma E, Doudna J. RNA-programmed genome editing in human cells. eLife. 2013;2. doi:10.7554/elife.00471
- Moon SB, Kim DY, Ko JH, Kim JS, Kim YS. Improving CRISPR Genome Editing by Engineering Guide RNAs. Trends Biotechnol. 2019;37(8):870-881. doi:10.1016/j.tibtech.2019.01.009
- 1. Fidler B. Tome biosciences debuts with $213M and a new way to edit the genome. BioPharma Dive. December 12, 2023. Accessed December 20, 2023. https://www.biopharmadive.com/news/tome-biosciences-gene-editing-startup-programmable/702092/.
- Next-level quality control in cell and gene therapy. BioPharma Dive. July 25, 2022. Accessed December 20, 2023. https://www.biopharmadive.com/spons/next-level-quality-control-in-cell-and-gene-therapy/627179/.