Most protein purification workflows require desalting, buffer exchange, or both at one or more steps. But how do you determine which method to use? And how do you choose the right product for your needs? Learn more in Emma’s blog post, where she answers some questions about using size exclusion chromatography (SEC) for this purpose.
When should I desalt my protein sample or change the buffer?
Perform this step whenever salt might cause problems in the next step or the sample needs to be in a different buffer. It can be incorporated before, during, or after purification or prior to analysis. For example, desalting and/or buffer exchange is typically performed in the following instances:
Before purification: to get the sample into a suitable buffer to ensure that your target protein binds to the column
Between purification steps: before ion exchange (IEX) chromatography, to decrease the ionic strength so proteins can bind to the column
After purification: to neutralize the pH of antibodies eluted from an affinity chromatography (AC) column or to adjust the conditions of the final purified protein
Before analysis: to remove excess salt that can cause wavy lanes or streaking during electrophoresis or can lead to poor labeling efficiency in Western blotting
What are the advantages of desalting columns compared with dialysis?
Many scientists still use dialysis for desalting and buffer exchange. Although it’s effective and inexpensive, dialysis can be time consuming and requires large volumes of buffer relative to sample volume. With dialysis, there is also the risk that material and target protein activity will be lost during handling.
Dialysis can take several hours to overnight, and multiple buffer changes may be necessary. In contrast, size exclusion chromatography (SEC; also called gel filtration) is quick, often taking just minutes with good recovery. The use of prepacked SEC columns for desalting minimizes preparation time, further adding to the time savings over dialysis.
What is Sephadex resin?
GE offers many types of Sephadex resin (based on dextran), which has been a mainstay in desalting and buffer exchange since the 1950´s. For most applications we recommend Sephadex G-25 or G-10. Use G-25 if your protein of interest has a molecular weight > 5000 and G-10 for smaller molecules with a molecular weight > 700. Loose resins are available, but we suggest prepacked formats for time savings, ease of use, and reproducibility.
How much sample can I load onto my desalting column?
The most critical parameter to ensure effective desalting is the sample-to-resin volume ratio. To minimize dilution and still retain good separation, we recommend a sample volume that does not exceed 30% of the total bed volume.
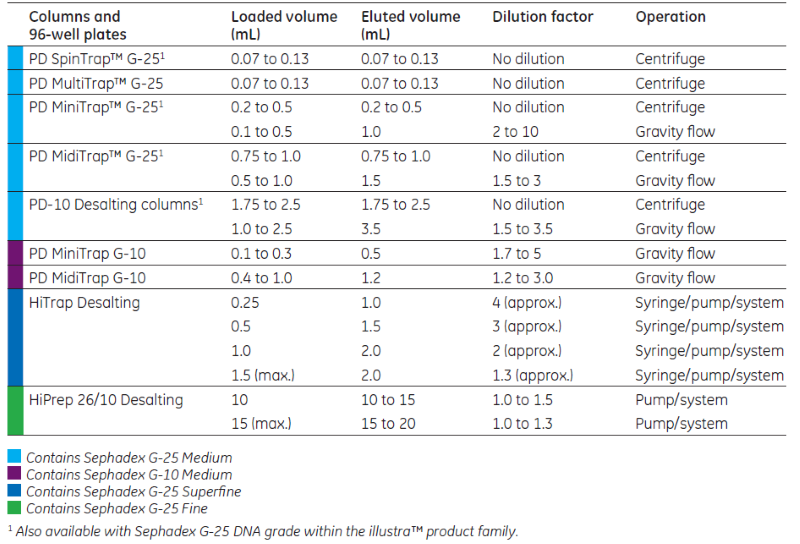
How do I choose the right prepacked column for my needs?
When choosing a prepacked column or plate, consider your protein’s molecular weight, your sample volume, the desired throughput, and the method or equipment you plan to use. Learn more here or let GE help you find the right prepacked desalting column for your application.

Do you have any more tips to optimize desalting?
Desalting columns can be incorporated where they are needed in a protein purification workflow. However, it’s a good idea to keep the number of steps in a protein purification process to a minimum, because each added step can decrease recovery. Careful design of a purification process can help to ensure that your final protein has the desired purity and yield. Note that if you need to have a SEC step in your protocol for other reasons, desalting/buffer exchange is already “built in.”
Giving some extra thought to the format, resin choice, and number of purification steps when using desalting columns can make a big difference in your protein purification results.