The polyhistidine-tag is the most widely used affinity tag in research involving recombinant proteins. It is small, and hence, the first choice in many protein purification workflows. Purification of his-tagged proteins is often straightforward using an immobilized metal ion affinity chromatography (IMAC) resin. Still, low or no recovery of your target protein can occur.
Purification of his-tagged proteins
Even though purification of his-tagged proteins using IMAC is considered relatively easy, it is not completely free from challenges. From time to time, recovering your target protein can be a real pain.
When working with eukaryotic systems such as mammalian or insect cells, from which the expressed his-tagged protein is secreted directly into the cell culture medium, low recovery from the IMAC step can sometimes be experienced. The low protein recovery is often related to incompatibility between the IMAC resin and the cell culture medium. The cell culture medium can contain EDTA and other components that will strip the immobilized metal ions from the IMAC resin during sample loading. The situation might be even further complicated by a low concentration of your target protein. Obtaining sufficient amount of pure protein would require large sample volumes (> 5 L), which would cause increased metal ion stripping.
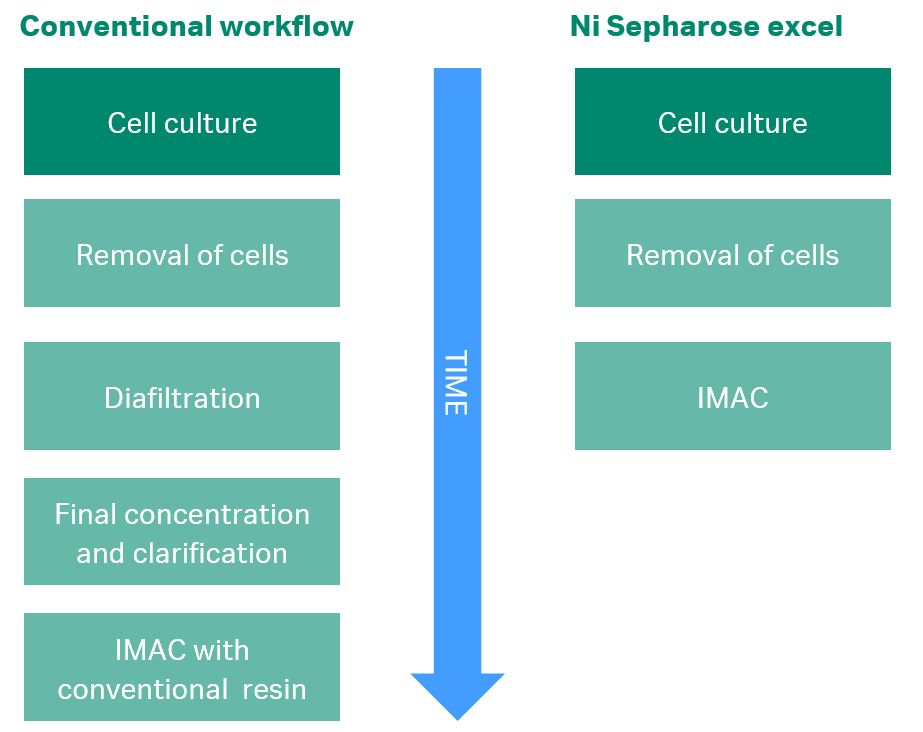
One solution to this problem is to prepare your sample prior to loading on the IMAC column. Sample preparation can comprise clarification and concentration of your sample in combination with a buffer exchange step. This pretreatment is time consuming and can have a negative effect on sensitive proteins.
Another solution is to use our Ni Sepharose excel resins, or the corresponding prepacked column HisTrap excel. The nickel ions are so strongly bound to this IMAC resin that samples, which normally would cause stripping, can be loaded onto the resin without pretreatment. The nickel ions have been shown to remain bound to the resin in the presence of as much as 100 mM EDTA. Ni Sepharose excel resin will save you loads of time, while preventing degradation of your sensitive protein.
If you experience low recoveries due to nickel-stripping using conventional IMAC resins, Ni Sepharose excel resin enables efficient and simplified workflows for capture, purification, and reproducible screening of his-tagged proteins expressed in various cell types, including both prokaryotes such as E.coli as well as eukaryotic cells such as insect or Chinese Hamster Ovary (CHO) cells.
Read more about His-tagged proteins in the Knowledge library.
Learn how to set up a purification protocol for His-tagged proteins