Each step in a diagnostic test’s journey—from raw material extraction to patient administration—can influence outcomes, demanding security of supply across the entire supply chain.
The need for supply chain solutions in the age of precision health
The recent increase in the development of biological diagnostic tools, as well as biological drugs, has reinforced the demand for heightened material controls. As advances in genomics work their way into the diagnostics market, manufacturing and material handling are more integrally tied to assay accuracy than ever before.
The use of living cells in the production of biologics—whether for research, diagnostics or patient-specific therapies—requires close monitoring of environmental stresses to promote optimal conditions for cell growth. Material purity, transparency, and traceability are needed to maintain optimal conditions for biological processes with narrow tolerance windows. Over time, industry understanding of the effects of raw material variability and the presence of trace elements on cell lines has made this a critical area of focus.
When available and disclosed, raw material origin and trace element compositions are useful indicators to predict performance or modify production. A thorough understanding of raw material characteristics and their effects on key product metrics will provide consistently higher titers and yield, guarantee quality, and control costs.
This deep level of understanding is something we strive for within the current global supply chain. Proposed strategies to reduce supply chain risk are regularly evolving but have yet to be perfected or universally adopted. In today’s high-stakes era of rapid point-of-care (POC) testing and personalized medicine, securing incoming and outgoing supply is a challenge faced by all players in this industry. This obstacle can only be overcome with an industry commitment to learning from the past and from one another to develop and adapt future supply chain solutions.
To maintain the bench-to-bedside continuum, improve product reliability, and save lives, companies must critically examine their supply chain management strategies and act to mitigate exposure to adverse supply events. Qualifying two suppliers, implementing a scorecard methodology for strategic suppliers, and surveying suppliers for numbers of production lines, sites, or facilities serve as a worthwhile starting point, but opportunities remain to further reinforce supply chain operations.
The Genomics and Diagnostic solutions supplier risk management program
High quality products which deliver reliable and consistent performance start with a reliable, secure supply of consistent raw materials. To achieve this, we take a proactive approach to security of supply, from a comprehensive raw material supplier risk management program and risk mitigation plans, through to rigorous product release testing based on agreed QC specifications to support performance claims.
Through our supplier risk management program, we:
- Reduce the number of high-risk suppliers.
- Mitigate risk through improved knowledge and assement of potential incidents.
- Use the most resource effective activities to reduce supplier risks.
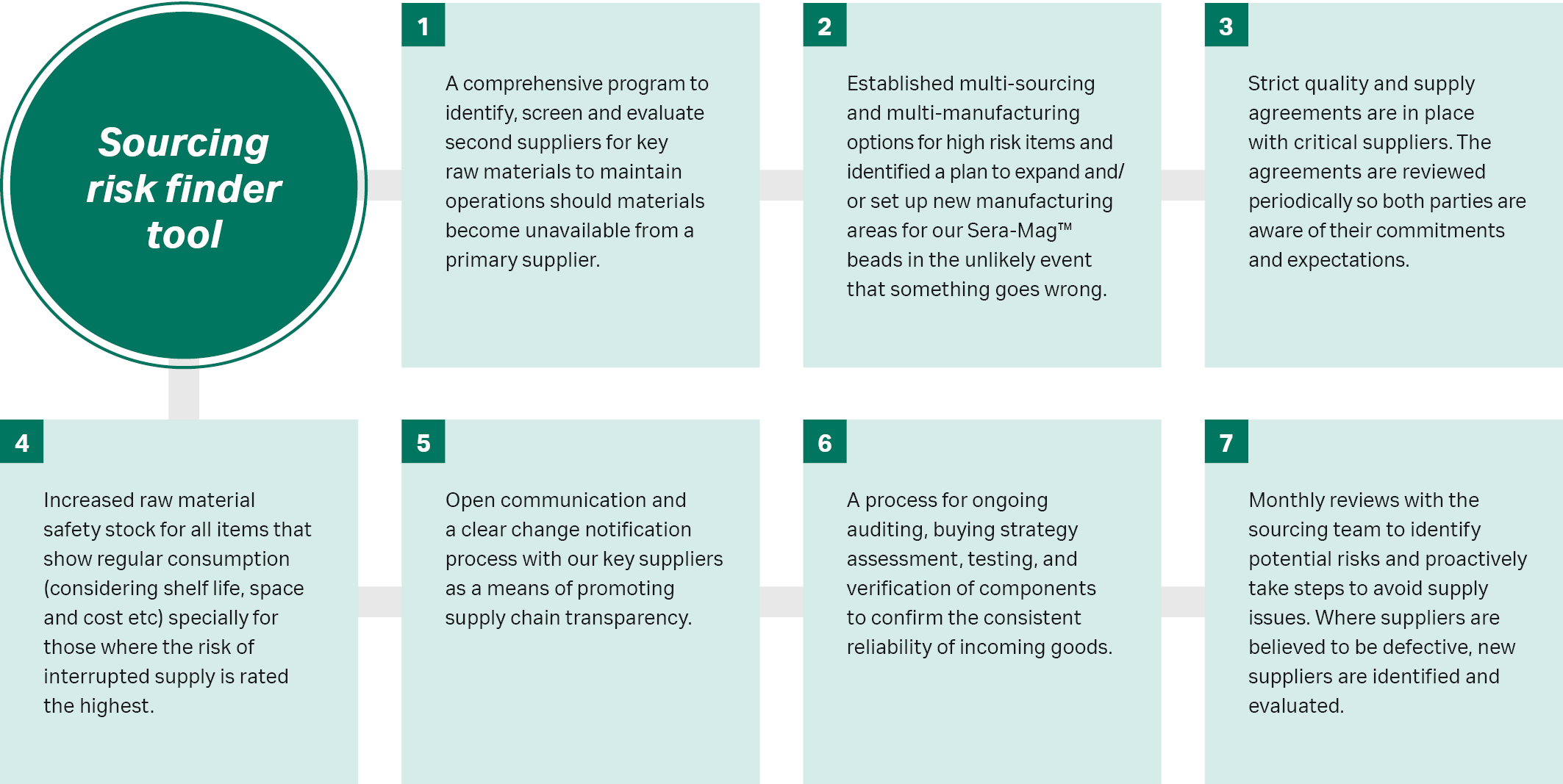
As part of our supplier risk management program, we have identified high risk items and established multi-sourcing and multi-manufacturing options for these items. We have established a sourcing risk index (SRI) to identify supplier and component risk and developed a sourcing risk finder tool to assess supplier lead times, relationships, safety stock and processes. We have implemented a comprehensive program to identify and evaluate second suppliers for key raw materials, so we can maintain operations should materials became unavailable from a primary supplier.
Strict quality and supply agreements are in place with critical suppliers. The agreements are reviewed periodically so both parties are aware of their commitments and expectations. Raw material safety stock for all items that show regular consumption is closely monitored and managed with considerations for shelf life, space and cost to ensure continuity of supply.
All of this is made easier through open communication and a clear change notification process with our key suppliers and to customers as a means of promoting supply chain transparency.
Supplier risk management is a strategic practice
Effective risk assessment and mitigation strategies require a cross-functional, data-driven commitment to supplier risk management. We take a proactive and strategic stance to supply-chain risk management to ensure that we are prepared to react quickly and decisively to unforeseen events. By assessing risk in advance, we can determine and plan for the potential impact of raw material variability and availabilty. Having identified high-risk areas, we can prioritize and implement initiatives to address them. We continuously monitor the points susceptible to potential compromise, with an overarching goal of identifying unexpected issues quickly, facilitating a timely response, and reducing risk in a measurable and objective way.
Security of supply is integral to quality and availability
The supply chain ends with the patient, and patient safety is paramount. Diagnostic accuracy, drug purity and availability are crucial. A concerted security of supply effort drives risk out of manufacturing operations that deliver quality products to market. Having a dedicated team focused on securing the supply chain increases accountability and guarantees actions will be taken to put safeguards in place. This bolsters product confidence for all stakeholders. In a system with solid foundations supporting security of supply, this process is collaborative and data-driven.
The supply chain of the future: it’s coming, and it’s digital
Digital supply chain developments in the pipeline are primed to transform supply chain logistics and supplier risk management. Moving away from static certificates of assurance to dynamic digital documents, including electronic Certificates of Analysis, will provide manufacturers with access to critical information prior to production. With quality data readily available, manufacturers can anticipate necessary changes and proactively adapt operations based on the characteristics of incoming raw materials. Additionally, the ability to track performance and analyze variable effects over time will increase knowledge and understanding of key metrics, advancing our ability to identify high and low risk areas
The safe delivery of diagnostic tools and therapies to market requires a cohesive effort across the entire supply chain. New knowledge, analytical capabilities, manufacturing technology, and industry consciousness are contributing to a more informed, transparent, and functional supply chain, but there is still much work to be done. Reducing overall risk and addressing the current limitations and challenges of supply chain management will take a concerted industry effort. As an industry, we must reach further than ever before to gain a true understanding of materials, supply chains, and their respective influence on biological therapies and diagnostic tools. This forward-thinking approach is a central element in supply chain risk management. With open communication and shared ideas for improvement, collaboration and continuous improvement will increase our collective success.