FAQ
Change Control Notifications and Regulatory Support Documentation are available for this product at the Regulatory Support Web Application, www.cytivalifesciences.com/rsf
After registering an account, you can start your subscription free of charge.
Packing procedures
| Column | Compression factor | Slurry concentration (%) | Bed height (cm) | Flow velocity (cm/h) Step 1 | Pressure (bar) Step 2 |
|---|---|---|---|---|---|
| BPG 100/500 | 1.15 | 30 - 50 | 10 - 30 | 20 | 6 |
| BPG 140/500 | 1.15 | 30 - 50 | 10 - 30 | 20 | 5 |
| BPG 200/500 | 1.15 | 30 - 50 | 10 - 30 | 20 | 5 |
| BPG 300/500 | 1.15 | 30 - 50 | 10 - 30 | 20 | 3 |
| Column | Maximum operation pressure (bar) |
|---|---|
| HiScale | 20 |
| XK 16 | 5 |
| XK 26 | 5 |
| XK 50 | 3 |
| BPG 100 | 8 |
| BPG 140 | 6 |
| BPG 200 | 6 |
| BPG 300 | 4 |
| BPG 450 | 2.5 |
| BioProcess LPLC | 6 |
| BioProcess MPLC | 20 |
| Chromaflow 400 | 3 |
| Chromaflow 600 | 3 |
| Chromaflow 800 | 3 |
| Chromeflow 1000 | 3 |
| FineLINE Pilot 35 | 20 |
| FineLINE 70 | 20 |
| FineLINE 100 | 20 |
| FineLINE 200 | 20 |
| FineLINE 350 | 20 |
| Bed height (cm) | Column diameter (mm) | Operating pressure of the column (bar) | Recommended prepacked column |
|---|---|---|---|
| 10 - 20 | 16 | 3 | 1-2 x HiLoad 16/101 |
| 20 - 30 | 16 | 3 | 2-3 x HiLoad 16/101 |
| 10 - 20 | 26 | 3 | 1-2 x HiLoad 16/101 |
| 20 - 30 | 26 | 3 | 2-3 x HiLoad 16/101 |
1 For higher bed heights than 10 cm run HiLoad columns in series
Two step packing technique: Constant flow packing followed by constant pressure packing.
Packing buffer for Butyl Sepharose High Performance and Phenyl Sepharose High Performance: Alcohol
Packing buffer for Q Sepharose High Performance and SP Sepharose High performance : Water
For more information regarding packing methods please read Column packing and preparation in Instructions Phenyl Sepharose and Butyl Sepharose High Performance.
The same method is valid for Butyl Sepharose High Performance, Phenyl Sepharose High Performance, Q Sepharose High Performance and SP Sepharose High Performance.
| BPG 100 |
|---|
| 10 or 12 µm nets |
| Adaptor net 10 µm Code no: 18-1103-05 |
| Adaptor net 12 µm Code no: 18-1103-06 |
| End piece net 10 µm Code no: 18-0251-77 |
| End piece net 12 µm Code no: 18-1104-41 |
| BPG 140 |
| 10 or 12 µm nets |
| Adaptor net 10 µm Code no: 18-1113-03 |
| Adaptor net 12 µm Code no: 18-1113-05 |
| End piece net 10 µm Code no: 18-1113-02 |
| End piece net 12 µm Code no: 18-1113-04 |
| BPG 200 |
| 10 or 12 µm nets |
| Adaptor net 10 µm Code no: 18-0252-76 |
| Adaptor net 12 µm Code no: 18-1104-42 |
| End piece net 10 µm Code no: 18-0252-77 |
| End piece net 12 µm Code no: 18-1104-43 |
| BPG 300 |
| 10 or 12 µm nets |
| Adaptor net 10 µm Code no: 18-1012-55 |
| Adaptor net 12 µm Code no: 18-1104-44 |
| End piece net 10 µm Code no: 18-1012-35 |
| End piece net 12 µm Code no: 18-1104-45 |
Packing in place technology
Refer to the document Pack-in-place using Chromaflow columns, method description and practical example, which you will find under the Related Documents tab. The same method is valid for Blue Sepharose 6 Fast Flow, Butyl Sepharose 4 Fast Flow, Butyl-S Sepharose 6 Fast Flow, Chelating Sepharose Fast Flow, CM Sepharose Fast Flow, DEAE Sepharose Fast Flow, Heparin Sepharose 6 Fast Flow, IMAC Sepharose 6 Fast Flow, Ni Sepharose 6 Fast Flow, Octyl Sepharose 4 Fast Flow, Phenyl Sepharose 6 Fast Flow (low sub), Phenyl Sepharose 6 Fast Flow (high sub), Phenyl Sepharose High Performance, PlasmidSelect, PlasmidSelect Xtra, Q Sepharose Big Beads, Q Sepharose Fast Flow, Q Sepharose High Performance, Q Sepharose XL, Sepharose 4 Fast Flow, Sepharose 6 Fast Flow, SP Sepharose Big Beads, SP Sepharose Fast Flow, SP Sepharose High Performance and SP Sepharose XL in Chromaflow columns.
Packing buffer: Water for Blue Sepharose 6 Fast Flow, Chelating Sepharose Fast Flow, CM Sepharose Fast Flow, DEAE Sepharose Fast Flow, Heparin Sepharose 6 Fast Flow, IMAC Sepharose 6 Fast Flow, Ni Sepharose 6 Fast Flow, PlasmidSelect, PlasmidSelect Xtra, Q Sepharose Big Beads, Q Sepharose Fast Flow, Q Sepharose High Performance, Q Sepharose XL, Sepharose 4 Fast Flow, Sepharose 6 Fast Flow, SP Sepharose Big Beads, SP Sepharose Fast Flow, SP Sepharose High Performance and SP Sepharose XL.
Packing buffer: 20 % Ethanol for Butyl Sepharose 4 Fast Flow, Butyl-S Sepharose 6 Fast Flow, Octyl Sepharose 4 Fast Flow, Phenyl Sepharose 6 Fast Flow (low sub), Phenyl Sepharose 6 Fast Flow (high sub) and Phenyl Sepharose High Performance.
Two step packing: Constant flow packing follow by constant pressure packing.
Packing buffer for PlasmidSelect Xtra, Q Sepharose High Performance and SP Sepharose High Performance : Water
Packing buffer for Butyl Sepharose High Performance and Phenyl Sepharose High Performance: 20% Ethanol
The same method is valid for Butyl Sepharose High Performance, Phenyl Sepharose High Performance, Plasmid Select Xtra, Q Sepharose High Performance and SP Sepharose High Performance.
Two step packning: Constant flow packing follow by constant pressure packing.
1. Pour some water (or packing buffer) into the column. Make sure that there is no air trapped under the bottom net. Leave about 2 cm of liquid in the column. Pour the gel slurry into the column.
2. Insert the adaptor and lower it to the surface of the slurry, making sure no air is trapped under the adaptor.
3. Seal the O-ring and lower the adaptor somewhat further into the slurry to fill the adaptor inlet with packing solution.
4. For maximal bed performance the bed is first settled at a low flow rate. e.g. 20 cm/h.
5. In the second step the flow rate is increased until a pressure of 3-5 bar is reached (depending on column). The column is run at this constant pressure for 45-60 min.
6. The adaptor is then lowered in steps. After each step, the same constant pressure of 3-5 bar is applied until the bed is stabilized.
7. Close the bottom valve, stop the pump, disconnect the column inlet and lower the adaptor approximately 3 mm into the gel bed without loosening the O-ring while the packing solution is flushing the adaptor inlet. If any air should be trapped here this can be removed by pumping liquid from the bottom (after inlet tubing and the bottom valve have been properly filled).
Recommended slurry concentration for packing of Sepharose High Performance media is 30-50%.
For packing long beds with Superdex prep grade, the slurry concentration can be increased to 70%.
Depending on the final bed height, a packing tube may have to be mounted on the column tube during the first part of the packing to avoid exceeding the critical slurry concentration.
| XK 16/40 |
|---|
| 10 µm nets (5 pieces/pack) Code No: 18-8761-01 |
| XK 26/40 |
| 10 µm nets (5 pieces/pack) Code No: 18-8760-01 |
| XK 50/30 |
| 10 µm nets (5 pieces/pack)Code No: 18-8759-01 |
| HiScale 16 |
| 20 µm nets (5 pieces/pack) Code No: 28-9663-79 |
| HiScale 26 |
| 20 µm nets (5 pieces/pack) Code No: 28-9663-80 |
| HiScale 50 |
| 20 µm nets (5 pieces/pack) Code No: 28-9663-81 |
| Column | Compression factor | Slurry concentration (%) | Bed height (cm) | Pressure (bar) |
|---|---|---|---|---|
| Chromaflow 400 | 1.15 | 50 - 70 | 10 - 30 | 3 |
| Chromaflow 600 | 1.15 | 50 - 70 | 10 - 30 | 3 |
| Chromaflow 800 | 1.15 | 50 - 70 | 10 - 30 | 3 |
| Chromaflow 1000 | 1.15 | 50 - 70 | 15 - 30 | 3 |
Recommended flow velocity interval in the table below is for packing the column. The interval is given in reverse order to indicate that the high flow velocity can be used for shorter beds whereas higher beds only allow lower flow velocity, due to the higher pressure drop across the bed.
| Column | Compression factor | Slurry concentration (%) | Bed height (cm) | Flow velocity (cm/h) Step 1 | Pressure (bar) Step 2 |
|---|---|---|---|---|---|
| XK 50/30 | 1.15 | 30 - 50 | 10 - 29 | 30 - 10 | 5.0 |
Packing small-scale AxiChrom columns
AxiChrom™ 50, 70, 100, 140, and 200 columns are designed to simplify column handling and maintenance during process development.
- Preprogrammed, verified packing methods that include Capto™, MabSelect™ media
- Reproducible, predictable and scalable results.
- Rotating pivot stand for easy emptying, maintenance and safer operation
- Proven sanitary design with construction materials recognized for use in GMP environments.
- Operates with ÄKTAexplorer™ and ÄKTApilot™ with UNICORN™ v 5.01 (and later).
AxiChrom columns are designed to simplify column handling and maintenance. Packing is facilitated by the Intelligent Packing methodology in the UNICORN software. The preprogrammed, verified packing methods shorten start-up time, eliminate errors and pack the column with optimal bed compression.
The pivot design allows the column tube to be tilted for convenient manual emptying as well as easy access to parts for maintenance.
Scalable and predictable performance over the entire AxiChrom platform is assured through the use of computational fluid dynamics in designing the liquid distribution system.
The AxiChrom column is sanitizable, and materials of construction are suitable for use in regulated environments. Comprehensive documentation, including IQ/OQ documents are available for all column sizes.
Packing large-scale AxiChrom columns
Large-scale AxiChrom™ columns are supported with Intelligent Packing and intuitive handling via the AxiChrom Master.The novel, swing-out-design facilitates maintenance and provides a safer working enivironment.
- Preprogrammed verified packing methods that include Capto™ and MabSelect™ media
- Reproducible and predictable results
- Swing-out, hoist-free design for easy maintenance and safer operation
- Proven, sanitary design meets stringent cGMP production standards
- Key steps guided by AxiChrom Master
Intelligent Packing provides preprogrammed, verified axial compression packing methods that promote accurate and reproducible results. Intuitive handling supports preparation and maintenance procedures. Both are controlled by the AxiChrom Master, a separate unit with a touch-screen interface that guides the operator through critical steps for priming, packing, unpacking, and maintenance.
Packing large-scale AxiChrom columns (Video below)
Easy access to all column parts for maintenance in-situ is facilitated by the swing-out, hoist-free, column tube design.
The distribution system design has been developed using analytical methods and modern computational fluid dynamic modeling tools to ensure reproducible and predictable results over the entire range of scales.
Materials of construction meet the ASME Bioprocessing Equipment Standard for biopharmaceutical manufacture.
AxiChrom columns are supplied with comprehensive documentation to facilitate set-up, installation, and operation.
Recommended net porosity for Butyl Sepharose High Performance, Phenyl Sepharose High Performance, Q Sepharose High Performance and SP Speharose High Performance: 10 µm
Column evaluation
The efficiency of a column depends on how well it is packed. A poorly packed column gives rise to uneven flow, resulting in zone broadening and reduced resolution. It is thus important to have a method by which the column can be tested before it is put into operation. Such a method should be simple, quantitative and should not introduce contaminating materials. It is also an advantage if the same method can be used to monitor column performance over its working life, so that it is easy to determine when the medium should be re-packed or replaced.
Avoid methods that use colored compounds such as Blue Dextran. They do not meet the above criteria and cannot be used with ion exchange and affinity chromatography media.
Experience has shown that the best method of expressing the efficiency of a column is in terms of the height equivalent to a theoretical plate, HETP, reduced plate number, h, and the peak asymmetry factor, As. These values can be determined easily by applying a NaCl or acetone solution, to the column (see below).
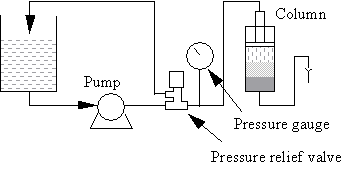
It is important that the column is properly equilibrated ( >2 column volumes) before evaluating the packing. Ideally, run three test runs to see whether the values are stable. If an initially poor result improves during a later test, the reason can be that the column was not properly equilibrated. To check that the bed is stable, run the column at 70% packing pressure for 20 hours and test it again.
Note that pressure spikes may cause poor packing (cracking). If this happens, fit an air trap and a pressure relief valve between the pump and column. Locate the pressure relief valve between the air trap and the column.
Choice of test sample for columns
The most appropriate material for column testing is, of course, the sample that is to be run in the application, but this is not always practical or economical. As an alternative, a solution of either NaCl or acetone will give a good indication of the column packing quality. The eluate is monitored by measuring conductivity or UV absorption, and the resulting elution profile is used to calculate the HETP value.
The advantages of using NaCl are that it is readily available and can be used safely to test all columns. One disadvantage is that NaCl may interact with the medium matrix, especially ion exchanger matrices, and thus give erroneous results.
Acetone, in contrast, does not interact with the matrix and is detected by UV absorption at 280 nm. Alternatively, you can increase the running buffer concentration 10-fold and use it as test solution.
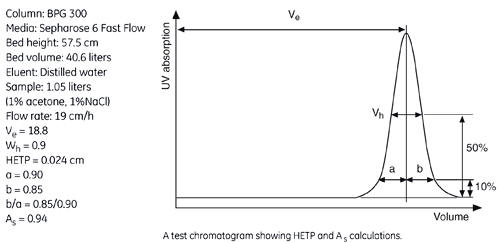
The figure below shows a UV trace for acetone in a typical BPG column application and gives calculated HETP and As values.
HETP calculation
The sample volume should be approximately 1% of the total bed volume and the concentration 1.0% v/v NaCl, or equivalent when using stronger buffer. Alternatively, use 1.0% v/v acetone. The flow velocity should be between 10 and 30 cm/h depending on the bead size of the chromatography medium. The high flow velocity could be used for small beads whereas large beads only allow low flow velocity. To avoid diluting the sample, apply it as close to the column inlet as possible. If an airtrap is included in the system, by-pass it during sample application to avoid back-mixing. Calculate the HETP value from the conductivity (or UV) curve as follows:
HETP, in its simplest terms, is expressed as:
HETP = L/N
where,
L = Bed height (cm)
N = Number of theoretical plates.
N is defined by the equation:
N = 5.54 (Ve /Wh)2
where,
Ve = Elution volume (ml)
Wh = Peak width at half height (ml)
Ve is measured as the volume passed through the column to the peak maximum.
Wh is measured as the peak width at half-peak height.
From the example in the figure, the HETP value can be calculated from the chromatogram as follows:
| Ve (ml) | Wh (ml) | N | N/m | HETP cm | |
| Acetone | 18800 | 900 | 2417 | 4203 | 0.024 |
Well-packed columns have low HETP values. However, it is only possible to compare columns that have been packed with the same type of media and that have been tested under identical conditions.
As a general rule-of-thumb, a good HETP value is approximately two to four times the mean bead diameter of the medium in question, provided that the sample does not interact with the medium.
In practice, the correlation between HETP and column performance can only be assessed by the column operator. Once this has been established, a standard can be set to judge the acceptability of a column packing.
For example, the column operator may know from experience that a column packed with Sephadex G-25 gel filtration medium with HETP values above 0.05 cm does not give the required separation. Consequently, the operator will set this value as the maximum permissible i.e. the minimum acceptable quality.
Reduced plate number
Definition of reduced plate number: h = HETP/dp
h = reduced plate number.
HETP = above described height equivalent to a theoretical plate.
dp = mean particle diameter of the chromatography medium beads.
The reduced plate number should be in the range of 2-4 times the mean particle diameter of the chromatography medium beads.
Peak asymmetry factor calculation
The peak asymmetry factor should be as close as possible to 1, and the shape of the peak should be as symmetrical as possible. This is usually the case for gel filtration media, but for certain ion exchange and affinity media, the shape may be asymmetrical due to interaction with the media. A change in peak shape is usually the first indication of column deterioration.
The peak asymmetry factor, As, is calculated from the graph above:
As = b/a
where,
a = distance from peak apex to 10% of the peak height on the ascending side of the peak
b = distance from peak apex to 10% of the peak height on the descending part of the peak
Note: Measuring HETP, h and As values is the best way to judge the condition of the packed column. A packed column can look good, but still need repacking for optimal performance. Always check the column after packing and regularly between runs to ensure best performance.
10 µm net stainless steel.