Being honest about what internal capabilities exist and whether the necessary skills and resources are available in-house to deliver the biomanufacturing strategy in a timely manner can reduce risk: speeding time to market .
When assessing your organization’s capabilities, you must be brutally honest about its resources and technical and capacity capabilities. Overcommitting in any of these areas can become a liability when you overextend. For example, you may have the technical capabilities but not the resources or the infrastructure, which could ultimately cause bottlenecks. External expertise in certain manufacturing strategies may also be required, such as in single-use solutions.
Biomanufacturing strategy: Capacity or capital, are you prepared?
One major risk to a company’s supply chain is demand volatility. Underestimating the demand of a product can lead to a drug shortage, preventing patients from receiving what can sometimes be life-saving medication. However, overestimating demand is just as risky, as this can lead to wasted inventory and even lost jobs. Nevertheless, a company must have enough capacity for its drug demand. The decision then becomes: Do you have the capacity and/or capital to control your own manufacturing destiny on your own, or do you need to engage the help of a contract development and manufacturing organization (CDMO)?
If you do not have the financial budget to support building a biomanufacturing facility or a bioprocess train, partnering with another company early can delay any CAPEX investments. Choosing a single-source partner that has access to not only end-to-end biomanufacturing capabilities but also to product development and innovations in biomanufacturing equipment, cell culture media, resins, and single-use devices can increase efficiency and speed to market.
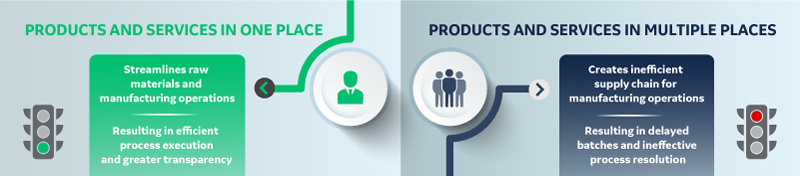
Strategic Biomanufacturing: In-house expertise, are you covered?
In addition, while small to midsize biotech companies may possess an arsenal of brilliant minds, they must have a full wing-to-wing understanding of media development, from discovery through commercialization and scale up. If that knowledge is not available in the early phases, the company will likely spend far too much time and money repeating experimentation. There could be issues later, too, if analytical assessments are not completed properly and the focus is on something like titer instead of on product quality attributes. With the right expertise, you can move forward very quickly with the design of your biomanufacturing process and then dovetail into other critical factors, such as cost of goods and availability of raw materials during scale up.
A thorough understanding of the availability of raw materials is also necessary, or you could find yourself in a situation where you are missing a critical component of your process. This triggers regulatory considerations, which may require additional clinical work to determine the impact. Having products and services in one place with a single-source provider can facilitate troubleshooting any raw material security of supply issues. There are various types of requirements for import and export, as well as trade barriers in some regions of the world that could affect availability.
Biomanufacturing regulatory considerations: Are you compliant?
The nuances between regulatory bodies across the world are often overlooked when determining a company’s ability to work on its own. A formulation has to not only be scalable but also accepted (and approved) by the country’s governing body, whether that be in the U.S. or in an emerging market, such as China. The latter is a particularly challenging area, as it does not allow clinical data to be leveraged globally. Instead, it must be performed again within the country, which can add three to six years of work. You must understand the impact any changes during development can have on not just the final formulation for your drug but also its eventual approval. Compliance should be considered as early as drug discovery, especially in biologic products, as the first-to-market company is the one that ultimately ends up owning that market space. Other companies that follow are at a disadvantage and must then adopt a new strategy, such as discounting their product, in order to be successful.
Thinking about working with a CDMO to complement your in-house resources? Read this article to identify opportunities where parallel operations can help you double up with a CDMO to reduce risk.
About Fast Trak Services
Cytiva has Fast Trak service centers in the USA, Sweden, India, South Korea and China and satellite Fast Trak Centers in Turkey, Japan and Singapore. The centers are specifically designed to help biopharmaceutical manufacturers increase their process productivity, reduce cost and enable them to bring their product to market faster. Contact us for more information.