A recent paper on trends in the biopharmaceutical market points to niche drugs for smaller patient populations, an intense focus on areas such as immuno-oncology, and more biosimilars as drivers for growing competition among companies targeting the same profit pool (1). As a result, biomanufacturers are looking for ways to improve process efficiency to cost effectively reduce risk in an increasingly fragmented global market. Process efficiency can be achieved in several ways, but you must ensure any changes to your process do not impose unnecessary restrictions that impact product quality, add complexity, or increase project timelines.
The goal is to use as few steps as possible to reach the ultimate industry goal—reliably manufacturing a safe, effective, high-quality drug at a price point that makes sense. Doing so requires a deeper look at how to streamline operations to continuously achieve maximum utilization and reduce waste without sacrificing quality in the race to be first to market.
Single-use supply management and the critical role of your supplier
When using traditional stainless-steel equipment, supplier engagement is limited to selling the equipment and offering the services needed for proper installation and operation. For example, it may be necessary to complete preventive maintenance or train the customer on how to optimally use the equipment they purchased. The customer-supplier relationship becomes vastly different once you make the move to single-use technology (SUT), as the overhead tasks and risks are now shared by the customer and the supplier.
While the customer must provide the necessary forecast of what they will need, the supplier must be able to consistently produce and provide any and all single-use consumables needed for the customer’s production batch, such as tube sets, bags, connectors, and sensors. One can try to mitigate the risk of running out of critical consumables when they most need them by storing or stocking significant inventory at the customer site; however, storing large inventories may negate the benefit of moving toward SUT in the first place. Instead of a limited transactional relationship, the customer-supplier relationship now evolves into a symbiotic partnership, dramatically changing the paradigm of engagement between a customer and their supplier.
To ensure the reliability and cost-effectiveness of single-use drug manufacturing is not compromised, customers must understand and audit the capabilities of their single-use suppliers (SUS). This includes supply chain sustainability, business continuity management systems, and appropriate sub-supplier sourcing and quality management systems. Any supplier they use must be compliant and credible and have the capability and capacity to provide appropriate security of supply. Cost of consumables becomes less important as security of supply becomes the most essential factor in preserving daily drug production operations and continued supply to patients in need. Many of the benefits of SUT are nullified if you do not trust your supplier from a supply chain and quality standpoint. Biopharmaceutical production can be unpredictable, so single-use consumable demands may vary as time goes on. Suppliers that can successfully manage spikes and drops in production volume will survive in an industry that continues to evolve and grow.
Selecting the right single-use supplier
Obviously, one goal of process efficiency is to find ways to do more at a lower cost. However, price should not be the only factor when choosing a SUS. It is imperative to choose a reputable company that has the appropriate quality systems, validation guides, and testing in place to demonstrate that the equipment and consumables are fit for purpose. There must be an assurance of security of supply as you move through product development, scale-up, clinical production, validation, and commercialization. What is the supplier doing to make sure they can provide you with the equipment and consumables you need on time, every time? The reliability they offer must go beyond just a marketing pitch; it must be something they truly believe in, and they must have the infrastructure and systems in place to provide consumables reliably and without quality issues.
Understanding change control notification procedures is another key factor when choosing a SUS. When material changes are made, SUSs need to have the appropriate change control notification programs in place. Customers need to be notified in a timely manner so they are aware of the changes and can complete the required product impact assessments. Late change control notifications could result in customer dissatisfaction, production delays, and scrapped production batches.
Another consideration is supplier and sub-supplier engagement. In most cases, SUS receive components from other suppliers, and if those relationships are not strong, it will limit the ability of the SUS to deliver a final product that consistently meets specifications. For example, suppliers should have knowledge about extractables and leachables and the compatibility of the materials of construction used in their products. If there is open communication between the sub-supplier and supplier when these changes occur, your supplier can then identify any potential impact to customers.
Overall, selecting the right SUS comes down to experience and expertise. You want a supplier that will provide you with a high level of confidence and will function as a partner and not just a supplier. They will understand the importance of offering customer training and education on all components they provide. They will take the time to understand the process and review process flow diagrams in order to provide the customer with the appropriate consumables needed for production. The supplier should provide the customer with a complete single-use consumables bill of materials needed for the entire production batch. The supplier should also make sure the customer understands the volume of consumables needed per batch. This is to ensure the customer has the warehouse space necessary for storage. This information is critical for new facility builds, as it will result in appropriate sizing of the customer’s warehouse.
Building a strong customer and supplier relationship is essential for successful outcomes. A connected partnership with open and honest communication breaks down the silos so often prevalent in biopharmaceutical manufacturing, allowing highly skilled teams to work together and create strategic plans for long-term success.
Gain efficiencies in manufacturing with a buffer management strategy
The goal of today’s industry is to manufacture high-quality biological drugs effectively and, in doing so, requires many critical activities. In some cases, these critical activities are kept in-house because they are proprietary to the customer or to the process. In other cases, the customer simply wants more control over the process. However, it may not be necessary to do all of the activities in-house. You can rely on suppliers to provide you with the right type of material at the right time so you can focus on more essential production activities. Similar to the way an SUS provides customers with consumables, some suppliers can also provide process liquids (solutions and buffers) needed for production.
Some of today’s production processes require a large volume and wide range of process liquids, as customers use solutions and buffers in different ways based on specific recipes with different concentrations, conductivities, and pHs. For example, chromatography involves different types of solutions and buffers for equilibration, washing, elution, cleaning, and regeneration. You must know ahead of time which process liquid you will need and for what unit operation. Also, solutions and buffers must be prepared, stored, and delivered to the right areas of your facility in a timely manner. These factors have led to a large number and overall volume of process liquids needed in a typical bioprocess workflow, making buffer preparation and management one of the most resource-intensive activities in biomanufacturing. It requires a great deal of resources from materials management, logistics, and operational standpoints, especially for solutions and buffers stored in controlled and classified cleanrooms.
While it may make sense for some customers to prepare and store solutions and buffers in-house, others may look for a supplier to provide them with the needed process liquids. Similar to single-use consumables suppliers, outsourcing process liquids also requires a capable and reliable supplier, so do your due diligence in selecting one with the experience, expertise, capacity, and appropriate quality and security of supply necessary to meet your production needs. Outsourcing buffer preparation also creates other opportunities for efficiency, such as reducing the footprint of your facility and the burden on quality control (Fig 1).
Outsourcing preparation of stock solutions enables great time-savings and optimizes resource utilization
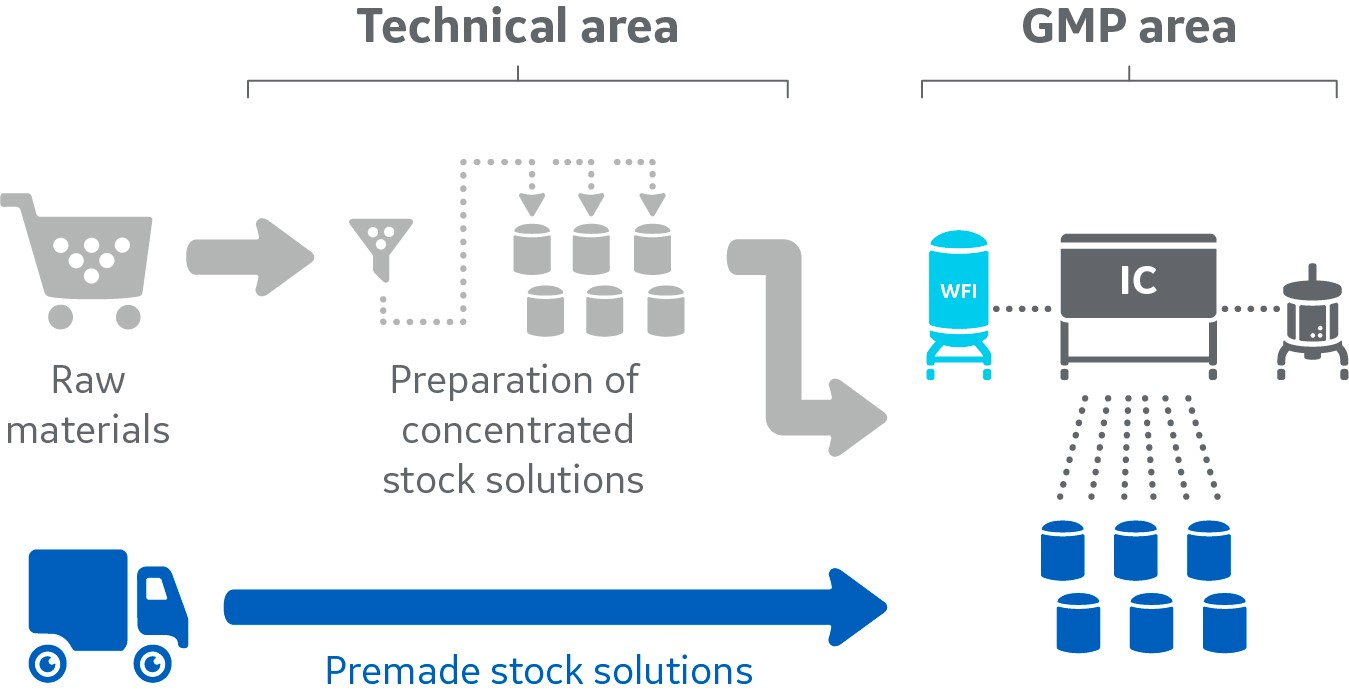
Fig 1. Outsourcing streamlines the extensive logistics needed to ensure that required raw materials are available just in time and qualified for a GMP setting.
There are other process liquid options available today for those that want to increase efficiency in this area. In-line conditioning or in-line solution and buffer preparation is another option, where the necessary components for the solution or buffer are automatically combined in real time at the point of use or in a buffer kitchen and transported to the unit operations in a single-use bag or storage vessel. Buffers and solutions could also be manufactured at the point of usage in a chromatography or filtration step. The process liquids are prepared using the specifications of the desired recipe. Any deviations that are detected by the system will be automatically adjusted or corrected utilizing a feedback strategy. This technology not only eliminates the need for solution and buffer storage, but it also minimizes manual operations and concerns about out-of-spec process liquids.
Taking advantage of today’s digital capabilities
Biomanufacturing of the future focuses on innovative solutions to meet the industry’s need for increased efficiency and speed. It includes the use of continuous processing, SUT, closed processing, flexible facilities, and data integration. An essential piece in connecting these tools and realizing the full capabilities of a “smart” facility is automation, which captures the data needed to analyze and optimize bioprocessing production. Regulators also recognize the value of data, as it can proactively identify potential issues and aid in investigating existing ones.
While automation serves as a key component of single-use, there is a perception in today’s industry that the single-use sensors and components used in closed processing — which reduce cleanroom classifications and the risk of contamination — do not have the same accuracy and sensitivity as those on clean-and-reuse equipment. Although this may have been the case a decade ago, single-use sensors have improved significantly since then, and these improvements have created opportunities for increased process efficiency and productivity.
An example of combining SUT and automation is Cytiva‘s integrated and configurable, single-use biomanufacturing platform, FlexFactory. Through a combination of distinct unit operations connected via single-use tubing sets and a variety of automation schemes, the FlexFactory platform can streamline production and increase flexibility and productivity. It enables a rapid response to changing capacity needs while maintaining compliance with regulatory requirements. FlexFactory is also the processing platform for GE’s KUBio turnkey modular facility solution. KUBio modular facilities are designed to reduce project timelines while incorporating the benefits of SUT. This proven solution utilizes GE’s bioengineers and global reach to develop configurable facilities that are supported by a suite of services. These types of platform and facility innovations create opportunities for process efficiency that not only increase speed to market and improve productivity but also mitigate many of the inherent risks of biomanufacturing.
Overall, as drug production becomes more diverse and complex, it is imperative to look for new ways to increase speed, efficiency, and flexibility while lowering your cost of goods. Strategies utilizing the latest technologies and solutions will provide the tools you need to improve throughput while continually responding to new challenges and changes in demand. In the end, these advantages may ultimately be the deciding factor between success and failure in this unpredictable but exciting market.
- Darby, Trends in the biopharmaceutical market: are you ready for the future of manufacturing? BioProcess Online (2018). https://www.bioprocessonline.com/doc/trends-in-the-biopharmaceutical-market-are-you-ready-for-the-future-of-manufacturing-0001. Accessed 23 July 2019.