Drug discovery programs are driven to process many samples efficiently so they can screen lead candidates quickly. Current methods to purify monoclonal antibodies (mAbs) often use liquid handling robots, columns packed with resins, and systems that can process samples in parallel. New technologies offer the opportunity for more flexible, fast, and efficient high-throughput methods. In this case study, AstraZeneca evaluates rapid cycling Fibro PrismA chromatography for high-throughput antibody purification in drug discovery and early process development.
About the lab
In a typical week, the Biologics Expression Team at AstraZeneca expresses and purifies up to 80 proteins at varied scale for studies in early-stage research. Part of the Antibody Discovery and Protein Engineering group, the team produces therapeutic proteins to support multiple projects across R&D.
Most of the proteins are antibodies and Fc-based, and they’re purified using column chromatography. Traditional column chromatography is limited by flow rates, and this can impact the lab’s throughput, especially when running larger volumes of proteins with poor expression. The lab would like to optimize their methods to produce high-quality proteins as quickly and efficiently as possible.
Table 1 summarizes the variety of samples the team sees and their current setup.
Table 1. Overview of samples, QC, and equipment in the biologic expressions team lab
| Molecule types | Volume range (mL) | Typical expression range (mg/L) | QC requirements | Systems and columns |
| • Antibody-based • Fc-tagged • Bispecifics • Fabs • His-tagged • Multi-species | 20 to 1200 | 35 to 500 | • OD280 • SDS-PAGE analysis • HP-SEC analysis • Endotoxin quantification | • ÄKTAxpress system* (13) • ÄKTA avant system (1) • HiTrap MabSelect PrismA and MabSelect SuRe columns (1 mL, 5 mL) • SEC and IEX as required • Other columns depending on molecule |
*Discontinued.
SEC = size exclusion chromatography.
Current challenges for high-throughput screening
Part of the team’s rationale for performing high-throughput expression and purification at the early research stage is to examine as many candidates as possible. They also conduct process development to find the optimal conditions for purification of those proteins.
The microscale cultures used in traditional high-throughput process development (HTPD) don’t always provide enough protein, because expression levels in early research are often very low. So, larger volumes must be cultured. Purification cycles with column chromatography take a long time, as flow rates are limited by diffusion-restricted binding. A typical purification cycle can take 55 minutes based on flow rates of 1 mL/min for a 1 mL HiTrap column on an ÄKTAxpress system.
Thus, purifying large numbers of proteins within a specific time frame can be a challenge and limits how many purifications the lab can do in a week. And that also limits the number of parameters they can look at in process development at any time.
Because of these factors, the team has scaled throughput by increasing the number of chromatography systems and connected HiTrap columns. The current setup for high-throughput antibody purification includes 13 ÄKTAxpress systems, which can run up to four, 1 mL HiTrap MabSelect SuRe or PrismA columns. This setup provides the lab with a maximum of 52 HiTrap columns to utilize in a working week.
Evaluation of Fibro PrismA units for high-throughput mAb purification
Fibro chromatography is based on an electrospun cellulose fiber matrix and has an open pore structure where mass transfer is governed by convective flow. Fibro PrismA consists of the PrismA protein A ligand immobilized to the cellulose base matrix. The combination of matrix and ligand allows for high mAb binding capacities at very short residence times, which results in cycle times of minutes instead of the hours needed for resin-based chromatography.
The Fibro PrismA units were an appealing option to Jen Spooner, purification specialist at AstraZeneca, because of this and because the robust cellulose fiber matrix can withstand the high pressures generated from increased flow rates allowing for much faster purification cycles. Combining this fiber matrix with the same robust purification ligand the lab already uses in the HiTrap chromatography columns ligand would potentially allow the team to perform antibody capture much more quickly and efficiently.
Head-to-head comparison with columns
Jen initially evaluated the Fibro PrismA units on ÄKTAxpress chromatography systems. The head-to-head comparison used two ÄKTAxpress systems: four 0.4 mL Fibro PrismA units were placed on the column positions of one instrument; and four 1 mL HiTrap columns were placed on a second instrument. Four 24 mL feed samples were loaded onto each instrument using the same program, except the flow rate was 1 mL/min for HiTrap columns and 16 mL/min for Fibro PrismA units.
Protein recovery and quality were comparable, but speed was much faster for the Fibro units – ~ 5.5 hours for 4 columns, and 55 minutes for four 0.4 mL Fibro PrismA units, which included a six column volume (CV) cleaning-in-place (CIP) and re-equilibration ready for the next run.
Based on these results, Jen estimated two staff members could purify the 80 protein samples, including larger-scale work and polishing steps, in 2.5 days instead of the 3.5 days it takes now. That’s a time savings of 1 day per week for each person. In addition, using the 0.4 mL Fibro PrismA units instead of 1 mL columns would only require 4 ÄKTAxpress systems for the smaller-scale purifications in a typical week, leaving 9 instruments available for other purification projects.
High-throughput purification on ÄKTA avant system
Another goal was to determine if Fibro PrismA units would be suitable for process development applications as well. The lab had recently purchased an ÄKTA avant system, which Jen coupled to a Teledyne™ ASX-560 autosampler (Fig 1). She designed studies to evaluate throughput and purification performance using a single 0.4 mL Fibro PrismA unit and compared the results to the lab’s standard workflow with MabSelect PrismA columns on ÄKTAxpress.
The setup was:
- ÄKTA avant and Teledyne ASX-560 autosampler
- One Fibro PrismA (0.4 mL) unit
- 100 samples run in batches of 10 to 50 samples
- CIP and equilibration between each sample
Fig 1. ÄKTA avant system, Fibro PrismA unit, and autosampler.
Purification time and performance were compared with the lab’s current setup by purifying a batch of 24 samples (human and mouse IgG) in duplicate on 6 ÄKTAxpress units and 24 HiTrap 1 mL columns. HiTrap MabSelect PrismA columns were used, because these use the same ligand as the Fibro units.
Purification cycle times for the Fibro PrismA unit were under 10 minutes/sample including CIP and re-equilibration of the Fibro unit as well as cleaning of the autosampler lines. The 24 samples were purified in approximately 3.5 hours using the single Fibro PrismA unit connected to the ÄKTA avant system with autosampler (Table 2).
On the existing setup using 6 ÄKTAxpress systems and 24 columns the purification time was approximately 5.5 hours. As a reference, given these purification cycle times it would take approximately 33 hours to purify 24 samples using a single HiTrap MabSelect PrismA column connected to an ÄKTA avant system and autosampler.
Table 2. Time comparison: Fibro PrismA on ÄKTA avant with autosampler vs current and reference setups
| Fibro PrismA unit | Current setup: 6 ÄKTAxpress systems + 24 columns | Reference setup: ÄKTA avant + autosampler + 1 column | |
| Time | 3.5 h | 5.5 h | 33 h |
These results demonstrate substantial time savings using Fibro PrismA units on ÄKTA avant with attached autosampler. In addition, only 1 instrument and 1 unit was used instead of the 6 instruments and 24 columns in their standard method.
Test for purification performance and robustness
Jen tested the robustness by running 100 samples on one 0.4 mL Fibro PrismA unit. Between each sample, the lab’s standard six CV CIP with 0.1 M sodium hydroxide was performed, followed by rinsing and re-equilibration, to ensure lack of carryover between samples and to keep endotoxin low. Table 3 summarizes the samples run on the unit, in addition to the 24 samples used for the ÄKTAxpress comparison.
Table 3. Samples run on one 0.4 mL Fibro PrismA unit
| Sample type | Replicates | Run how? |
| Control antibody, batch 1 | 13 | In succession |
| Control antibody, batch 2 (different titer) | 21 | Interspersed throughout the 100 runs |
| Bispecific | Duplicates of dilution series to simulate low expression level (90 mg/L; 60 mg/L; 30 mg/L) | In succession |
In addition to the samples in Table 3, various species of antibodies were run, as were different human isotypes. Then, in order to compare against the lab’s normal method, Jen’s expression colleague prepared twice the usual volumes of their smaller-scale expressions one week. Half of each sample was run on the Fibro PrismA unit attached to the ÄKTA avant system and autosampler; the other half was run on ÄKTAxpress with 1 mL HiTrap MabSelect PrismA columns. More control antibody was loaded onto the Fibro unit to finish off the 100 runs.
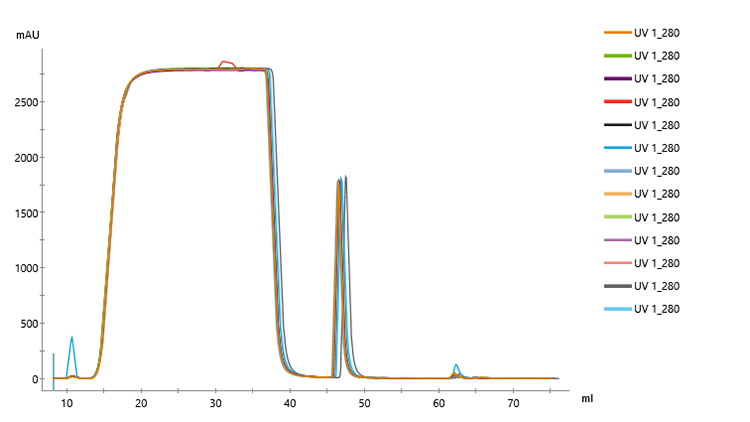
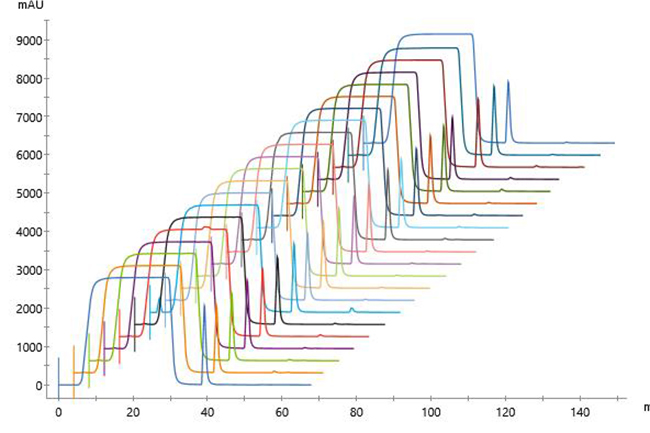
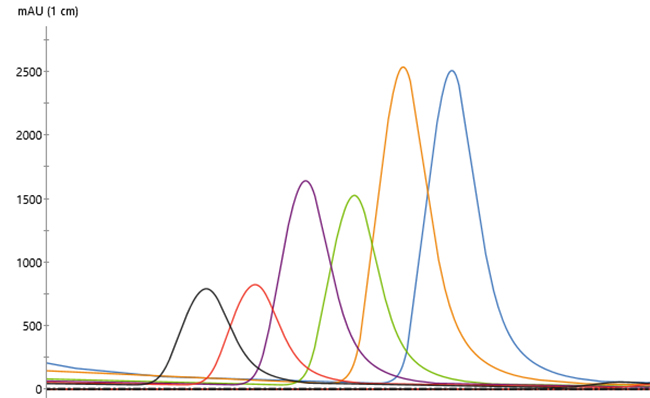
Overlaid chromatograms for 13 replicates showed reproducible performance (Fig 2). In addition, chromatograms for the 21 replicates were similar regardless of run number (Fig 3), indicating that column performance was not impacted by the CIP cycles between runs. Serial dilutions of the bispecific showed that concentrations down to 30 mg/L could be purified (Fig 4), which demonstrated that the Fibro PrismA units were suitable for the low-expression cultures the lab often handles. Purification of all species and isotypes was comparable to the standard column method. Finally, samples purified on Fibro PrismA showed comparable recovery and purity to samples purified on the 1 mL HiTrap MabSelect PrismA columns.
Fig 2. Overlaid chromatograms of 13 replicates of control antibody loaded onto a single 0.4 mL Fibro PrismA unit on ÄKTA avant with attached autosampler.
Fig 3. Chromatograms of 21 replicates of control antibody loaded onto a single 0.4 mL Fibro PrismA unit on ÄKTA avant with attached autosampler. Runs were interspersed throughout 100 cycles.
Fig 4. Chromatograms of a bispecific dilution series, each run in duplicate on a single 0.4 mL Fibro PrismA unit on ÄKTA avant with attached autosampler.
Summary and conclusions
Fibro PrismA purification on the ÄKTA avant system with attached autosampler took about 9 min per sample. Jen was happy with this time, given that she ran a full CIP and re-equilibration between each sample, and the autosampler lines had to be rinsed in between. Because the autosampler can handle 84 samples in succession, and the ÄKTA avant has six fraction collector plates, Jen says, “You set it up, and then you walk away.”
The main benefit to her lab? “It's the throughput, which means that we can look at more samples and do more process development on low-expressing proteins.” Jen adds, “The time savings is substantial, saving two staff one day each per week, so we can focus on more challenging work. It makes a big difference to us.”
Future directions
The lab has purchased their own autosampler and Fibro units, which they are using on a regular basis. In the future, Jen’s team would like to evaluate the larger Fibro PrismA units for their larger-scale purifications.
Acknowledgements
We thank Jen Spooner and her colleagues at AstraZeneca for insightful discussions and for allowing us to share their data.