HyClone VaccineXpress cell culture medium was used for the propagation of influenza virus and rotavirus in an animal-derived component-free (ADCF) process. Small-scale microcarrier cultures were optimized using spinner flasks by closely monitoring cell growth, viability, and morphology. The results were used for scaling up the process to a ReadyToProcess WAVE 25 bioreactor system at a 2 L working volume. The data show that the ADCF process using VaccineXpress for rotavirus propagation is scalable, promoting the production of infectious viral particles.
Introduction
There is an increasing demand for animal-derived component-free (ADCF) cell culture medium to be used in live vaccine production. To meet this demand we have developed and qualified HyClone VaccineXpress medium for high density growth and maintenance of adherent cell lines for viral vaccine development. The VaccineXpress medium is free of both serum and animal derived components. Furthermore, the chemically defined formulation of VaccineXpress improves reproducibility, while simplifying downstream purification of recombinant proteins and viruses in bioprocess applications.
To demonstrate that VaccineXpress medium is suitable for scalable cell culture applications, we performed low-volume cultures in flasks for the propagation of influenza virus and rotavirus. The process was then scaled up to demonstrate virus production using a ReadyToProcess WAVE 25 bioreactor system.
Materials and methods
Several cell lines are available for producing vaccines in cell culture-based systems, Vero cells from African Green Monkey Kidney cells (ATCC™ CCL-81™) are commonly used in the biopharmaceutical industry and were chosen for this study because of their suitability for use in producing human vaccines.
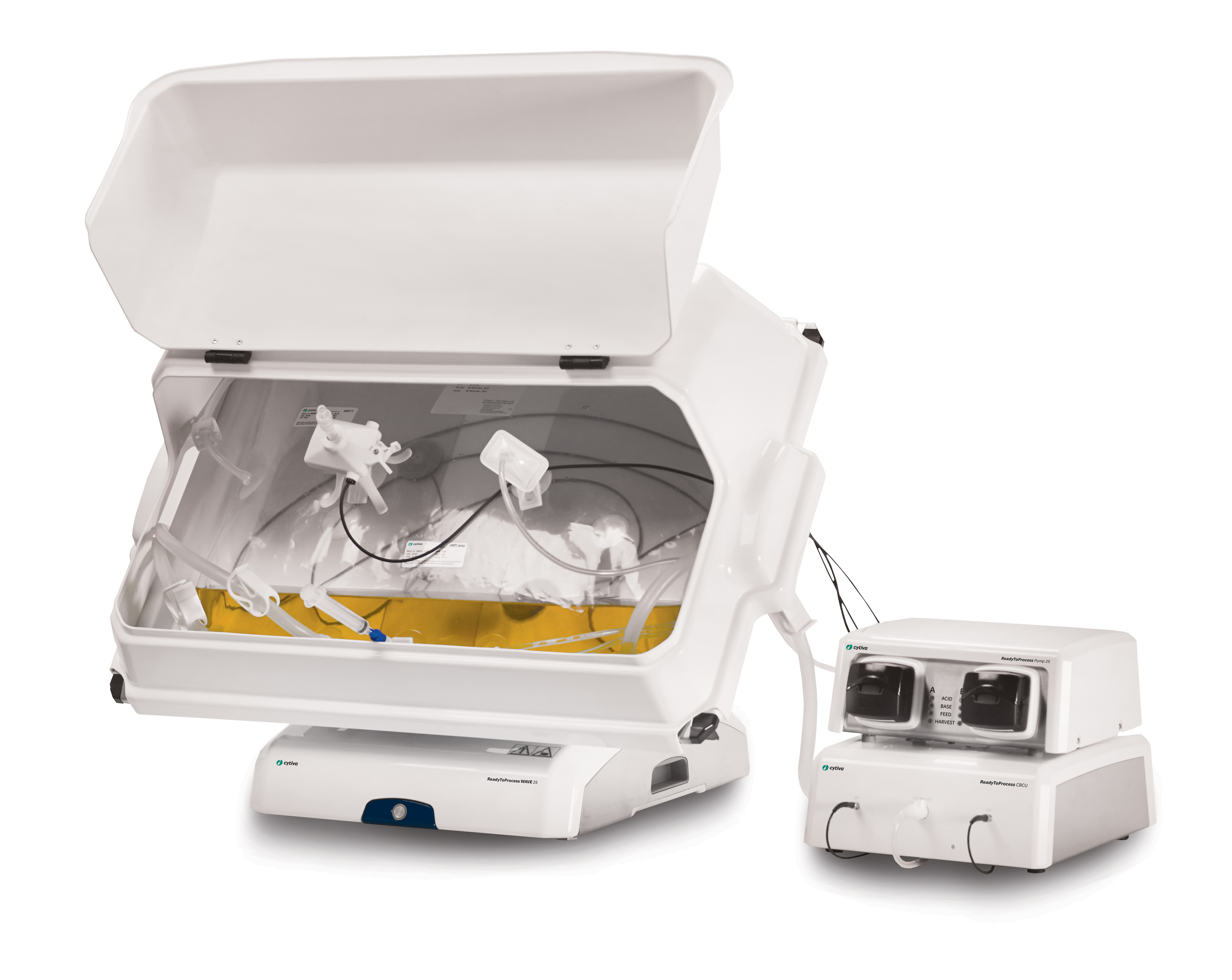
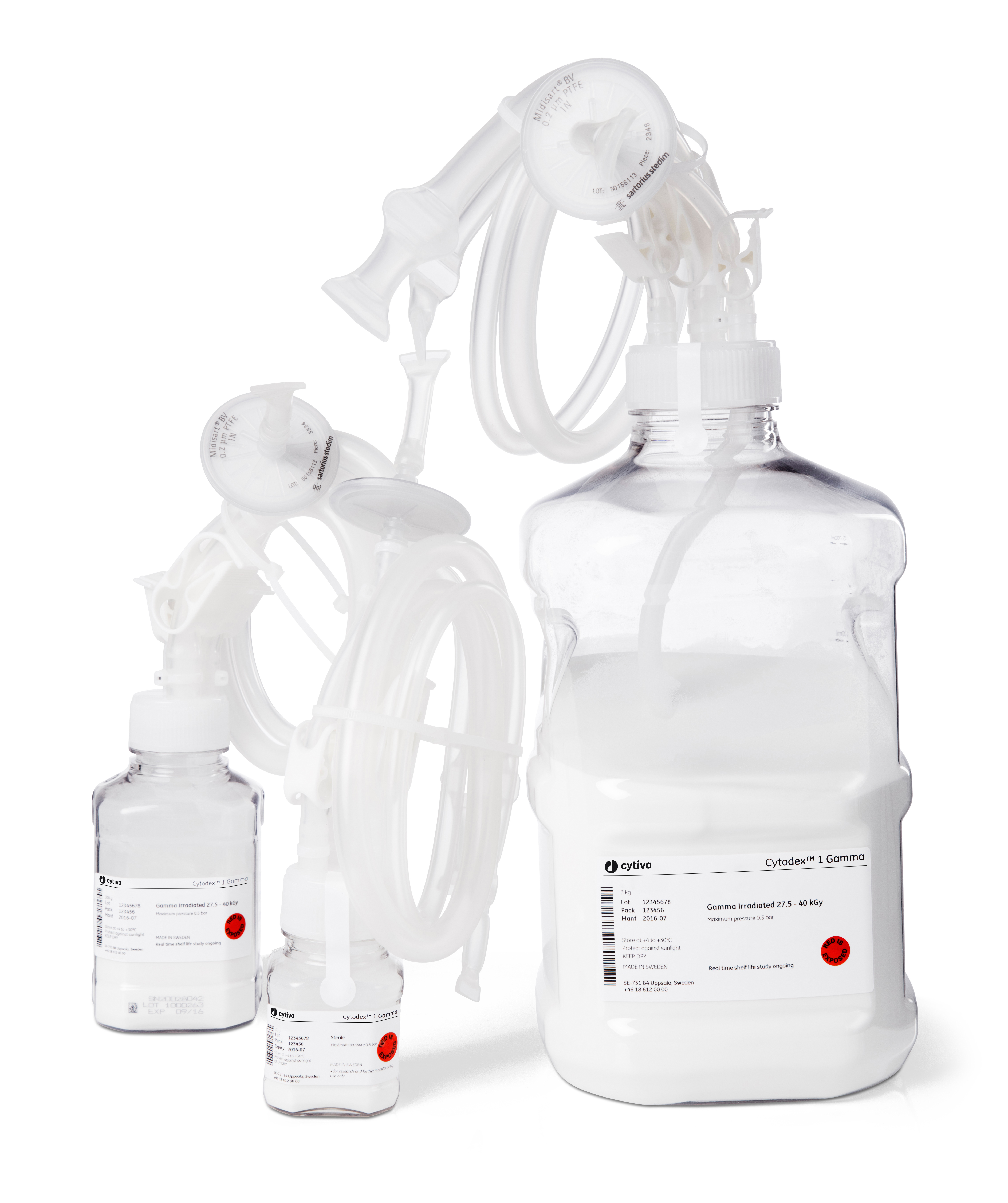
Virus production was performed in a WAVE 25 bioreactor system using single-use Cellbag bioreactor bags manufactured from Fortem™ film, Cytodex 1 Gamma microcarriers, and VaccineXpress cell culture medium (Fig 1). Sheffield™ rTrypsin ACF (Kerry, Inc.) was used for activation and propagation of rotavirus.
(A) ReadyToProcess WAVE 25 bioreactor equipped with Cellbag bioreactor containers
(B) Cytodex 1 gamma microcarriers
(C) VaccineXpress medium
Fig 1. Solution for virus production.
Maintenance, expansion, sampling, and counting
Vero cells were thawed and cultivated in VaccineXpress cell culture medium supplemented with 4 mM L-glutamine and cultured in T-flasks or Nunc™ Cell Factory™ systems (ThermoFisher Scientific). Culturing was performed at 37°C in a 5% CO2 environment. For maintenance and expansion culture, cells were washed with PBS-EDTA (Sigma-Aldrich) prior to addition of a recombinant protease for detachment, using either TrypLE™ Select (Invitrogen), Accutase™ (Sigma-Aldrich), or Sheffield rTrypsin ACF (Kerry). Trypsin inhibitor was added to the detached cell suspension and the cells were seeded at approximately 5 × 104 cells/cm2.
For routine culture, Vero cells were passaged in T-flasks twice a week and Nunc Cell Factory systems were used for cell expansion. For microcarrier cultivation, samples were taken daily to determine the cell growth, viability, and morphology. Cell concentration and viability were determined using a NucleoCounter™ NC-200™ cell counter (Chemometec).
Cell morphology and attachment to microcarriers was evaluated using an Eclipse TS100 inverted microscope with attached camera (Nikon).
Preparation of Cytodex 1 Gamma microcarriers for spinner cultures
Cytodex 1 Gamma microcarriers were used at a final concentration of 3 g/L. The required amount of microcarriers were hydrated for 2 h at 37°C in complete medium using a sterile glass or sterile Falcon™ centrifuge tube siliconized using Sigmacote™ siliconizing reagent (Sigma-Aldrich). The solution was then added to spinner flasks and equilibrated for 1 h at 37°C in 5% CO2 before cell inoculation.
Bioreactor cultures
A ReadyToProcess WAVE 25 bioreactor, connected to a ReadyToProcess CBCU controller, was equipped with a single-use 10 L Cellbag bioreactor inflated with air. The required amount of Cytodex 1 Gamma microcarriers was transferred directly into the disposable bioreactor and complete medium was added. The mixture, comprising VaccineXpress cell culture medium and Cytodex 1 Gamma microcarriers, was equilibrated for at least 2 h at 37°C and 5% CO2. An offset pH calibration was conducted before the cells were inoculated at a concentration of 0.4 × 106 cells/mL in a 2 L working volume. The culture was controlled at pH 7.1, 37°C, and 5% CO2. The rocking motion was set to 100% and pure oxygen was not used due to the short cultivation time.
At culture initiation, continuous rocking was set to 6 rocks per minute (rpm) at an 8° angle for the first 2 h and then increased to 10 rpm at a 6° angle. Two hours after seeding, a sample was taken to ensure that the cells had started to attach to the microcarriers. Thereafter, sampling was conducted every 24 h throughout the culture, to determine cell density and morphology. Prior to sampling, rocking speed was temporarily increased to 20 rpm for 1 min to ensure a homogenous solution. After 48 or 72 h, 50% of the working volume was exchanged for fresh culture medium.
When reaching a density of approximately 1 (± 0.2) × 106 cells/mL, the cells were infected with virus. Culture conditions were 37°C, pH 7.2, and 5% CO2.
Virus propagation
Influenza virus
Influenza A/Solomon Islands/3/2006 (H1N1) was used for infection. Prior to infection, the number of virus particles was calculated according to a multiplicity of infection (MOI) specific for the virus strain. Approximately 50% of the medium was removed from the cell culture and replaced with virus maintenance medium (VaccineXpress cell culture medium containing trypsin and virus) in the Cellbag bioreactor and the culture temperature was decreased to 33°C. After 1 h, prewarmed medium was added up to the 2 L working volume. The cultures were harvested when a cytopathic effect (CPE) occurred and analyzed for tissue culture infective dose (TCID50).
Rotavirus A
Rotavirus A (ATCC VR-2018), strain Wa (tissue culture adapted), derived from a patient positive for rotavirus, was used for infection. Rotavirus was incubated with Sheffield rTrypsin ACF (20 µg/mL) for 1 h at 37°C to activate the virus. In preparation for the infection, Vero cells were washed twice in PBS with Ca2+ and Mg2+ by letting the microcarriers settle. Medium was removed carefully to avoid accidental loss of any microcarriers. Following the second PBS wash step, activated rotavirus was added to the spinner flask/Cellbag bioreactor. Prior to infection, the amount of virus particles was calculated according to a MOI specific for the virus strain. The cells were infected for 1 h at 37°C and 5% CO2 at one-third of the total culture volume, under continuous stirring/rocking. After the virus had infected the Vero cells, the inoculum was diluted by adding VaccineXpress cell culture medium supplemented with Sheffield rTrypsin ACF (10 µg/ml) to support continued virus infection. The cultures were harvested when cytopathic effects occurred. Cultures were freeze-thawed three times at cycles of -80°C to +20°C, followed by low-speed centrifugation (2000 rpm for 10 min) to remove cellular debris from the harvest lysate. The clarified rotavirus was transferred into a new 50 mL conical tube and stored at -80°C. Rotavirus material was analyzed by ELISA for rotavirus detection and focus forming assays to determine the infectious virus titer.
Analysis
TCID50 assay
The TCID50 assay is an infectivity method. The outcome is presented as a titer (TCID50 units/mL), where 50% of the measuring units show cytopathic effects. Consequently, TCID50 is a measurement of the capability to initiate an infection. In short, Vero cells, grown in a 96-well microplate, are infected with 10-fold serial dilutions of a virus suspension. The microplate is incubated for 5 d, wells with cytopathic effects are counted, and the TCID50 titer calculated according to the method of Reed and Müench (1).
Rotavirus enzyme immunoassay (ELISA)
The rotavirus enzyme immunoassay is a quantitative method. The outcome is presented as a dilution factor, which is compared to a positive and negative control. The microwell plate is coated with a monoclonal antibody against VP6. This VP6 group is a specific antigen that is found in all rotaviruses that cause disease in humans. If rotavirus is present in the sample, a sandwich complex will be formed consisting of immobilized antibodies, rotavirus antigens, and antibodies conjugated with the biotin-streptavidin-peroxidase complex.
Focus forming assay
The focus forming assay (FFA) is an infectivity method using fluorescently labeled antibodies specific for a viral antigen to detect infected host cells and infectious virus particles. Rotavirus was serially diluted into a 96-well plate with African green monkey kidney cells (MA104 cells ATCC 85102918). After rotavirus infection, MA104 cells were labeled with an anti-rotavirus antibody and an FITC-conjugated secondary antibody. Plates were scanned with the IN Cell Analyzer 2200 and rotavirus infectious cells (green fluorescent cells) were counted using IN Cell Analyzer 1000 Workstation software. A titer of fluorescent focus forming units (FFU) per mL was then calculated.
Results
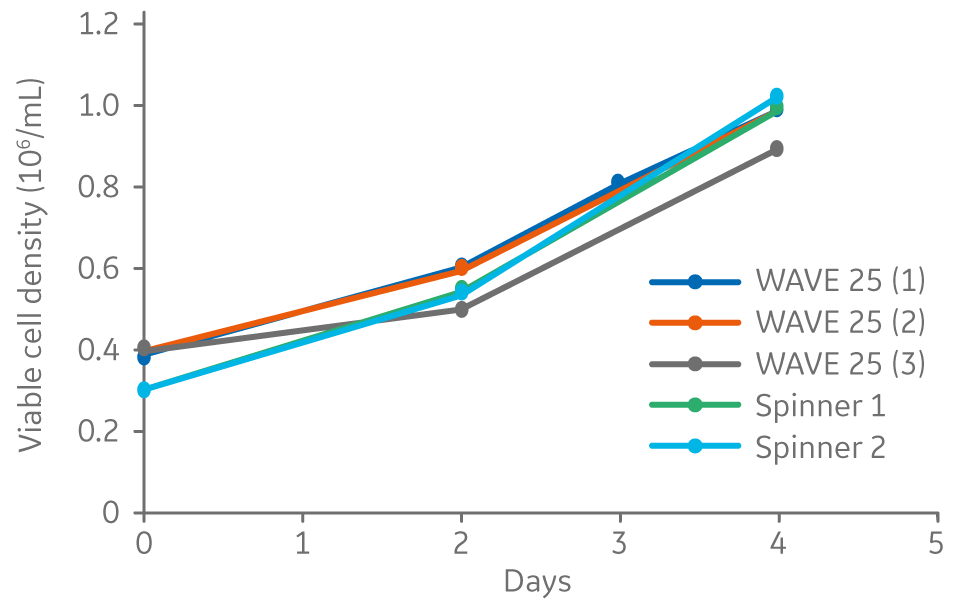
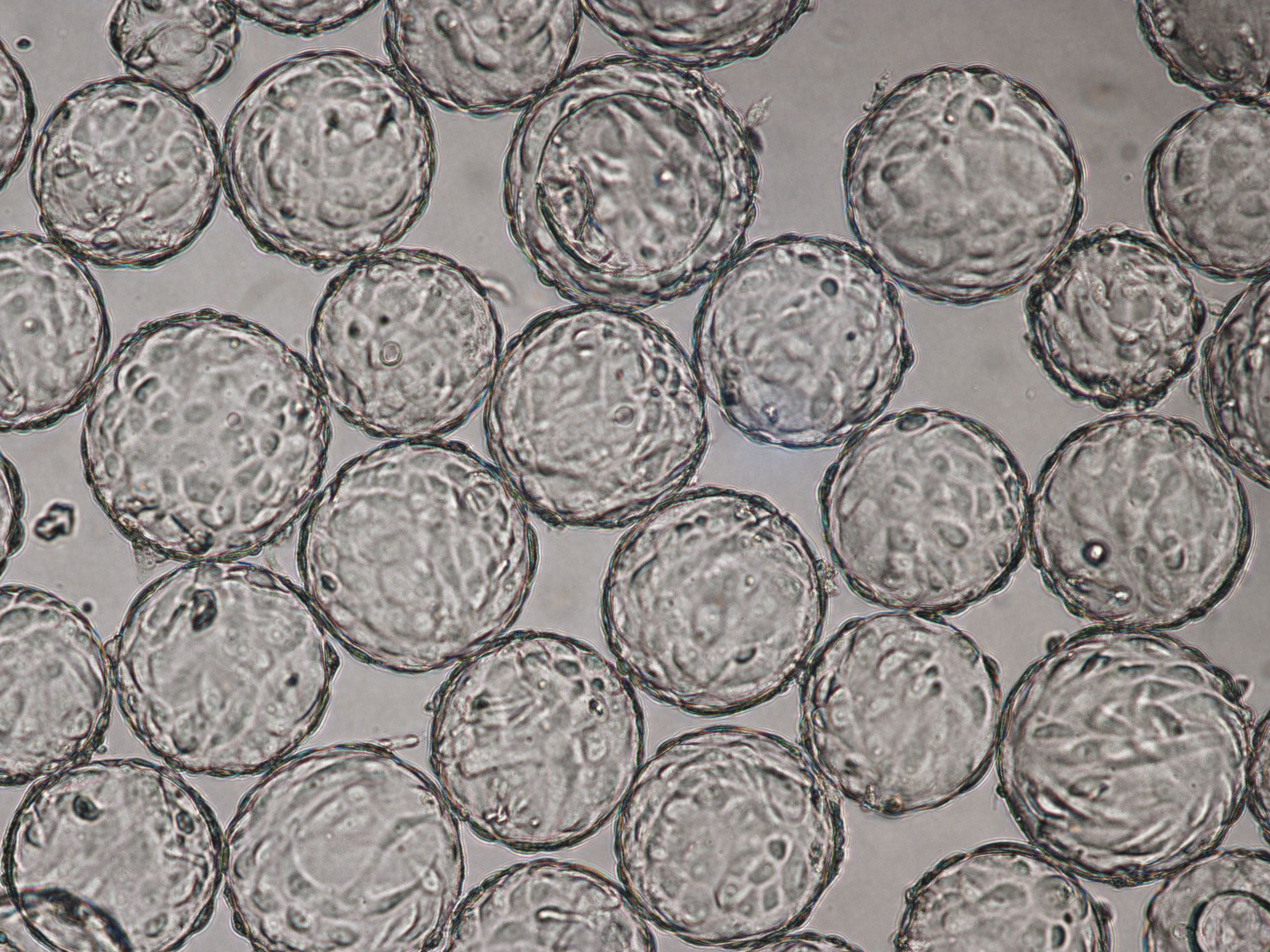
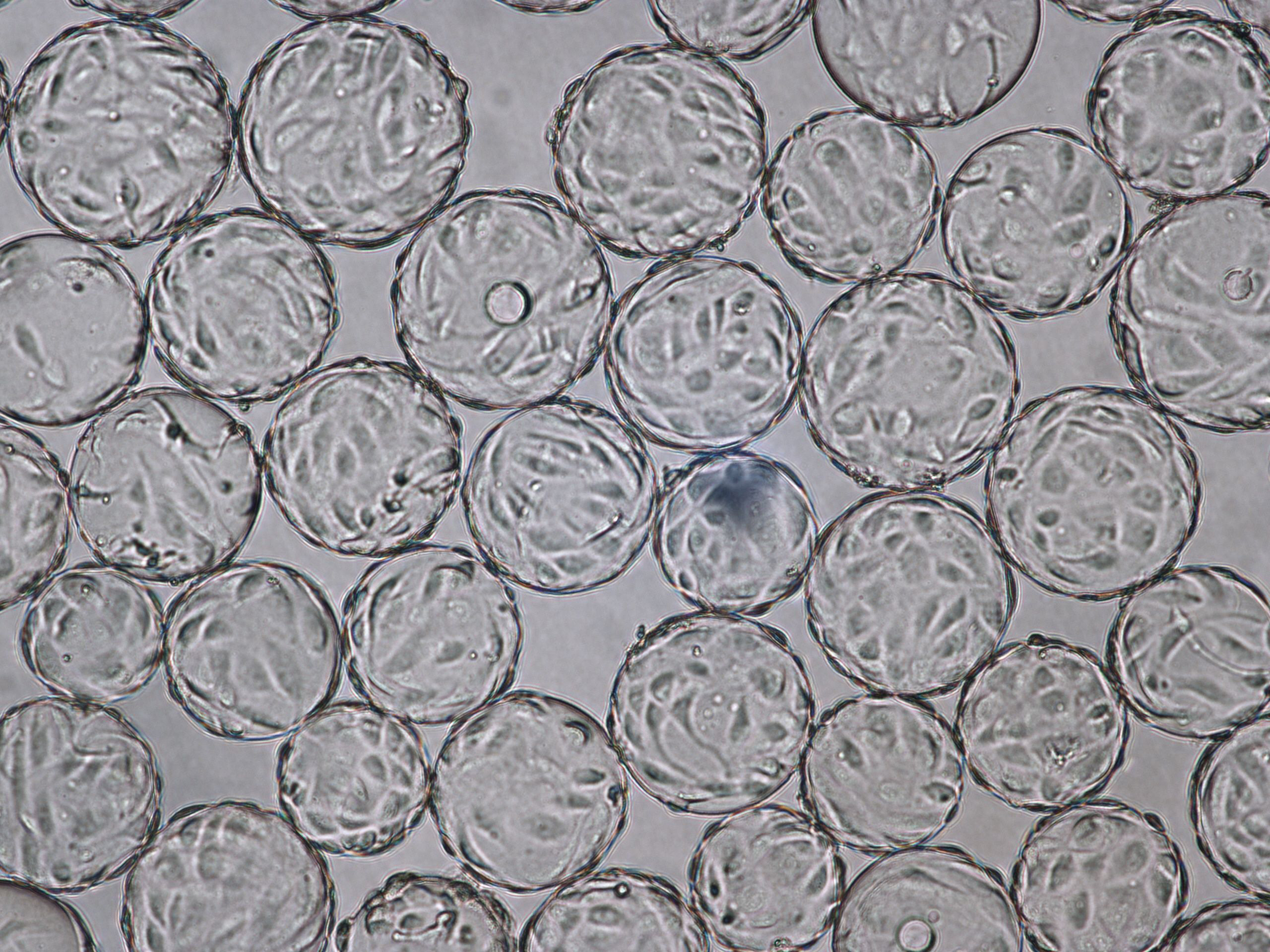
Vero cells cultured on Cytodex 1 Gamma microcarriers in VaccineXpress cell culture medium display similar growth rates and morphology in spinner flasks and in a ReadyToProcess WAVE 25 bioreactor (Fig 2 ).
Fig 2. Viable cell density for Vero cells cultured on Cytodex 1 Gamma microcarriers using VaccineXpress cell culture medium in spinner flasks and independent cultures in a WAVE 25 bioreactor.
Influenza virus
The morphology of Vero cells grown on Cytodex 1 Gamma microcarriers is shown at time of infection (TOI), which is after 96 h of cultivation (Fig 3). Cytopathic effects for influenza virus are shown at time of harvest (TOH, 96 hours post infection [hpi] Fig 4).
Influenza virus can be propagated in Vero cells cultured on Cytodex 1 Gamma microcarriers in VaccineXpress media in a ReadyToProcess WAVE 25 bioreactor, as shown by the TCID50 analysis for the two cultures (Table 1).
(A) 96 h cultivation (TOI) Vero cell culture in spinner flask
(B) 96 h cultivation (TOI) Vero cell culture in ReadyToProcess WAVE 25 bioreactor.
Fig 3. Comparable cell attachment and morphology of Vero cells on Cytodex 1 Gamma microcarriers using VaccineXpress cell culture medium in (A) spinner flask and (B) WAVE 25 bioreactor.
Fig 4. Cytopathic effect for influenza virus culture in WAVE 25 bioreactor at time of harvest (TOH), 96 hpi.
Table 1. TCID50 analysis results from two independent cultures of influenza virus propagation in the WAVE 25 bioreactor.
| Sample | TCID50 |
| Influenza WAVE 25 (1) | 107/mL |
| Influenza WAVE 25 (2) | 107/mL |
Rotavirus
Based on the positive results from culturing Vero cells and propagating influenza virus in the VaccineXpress media, we were interested in evaluating the performance of the media for propagating rotavirus in an ADCF process. An initial study was performed in spinner flasks using classical porcine-derived trypsin and recombinant trypsin. Trypsin of both sources promoted high rotavirus infectious titers, as measured by FFA analysis (Table 2) and IN Cell Analyzer (Fig 5).
Table 2. FFA results show that VaccineXpress promotes high rotavirus infectious titer using porcine trypsin as well as Sheffield rTrypsin ACF.
| Trypsin origin | Rotavirus infectious virus titer FFA (mL-1) |
| Porcine | 8 × 105 |
| Sheffield rTrypsin ACF | 5 × 105 |
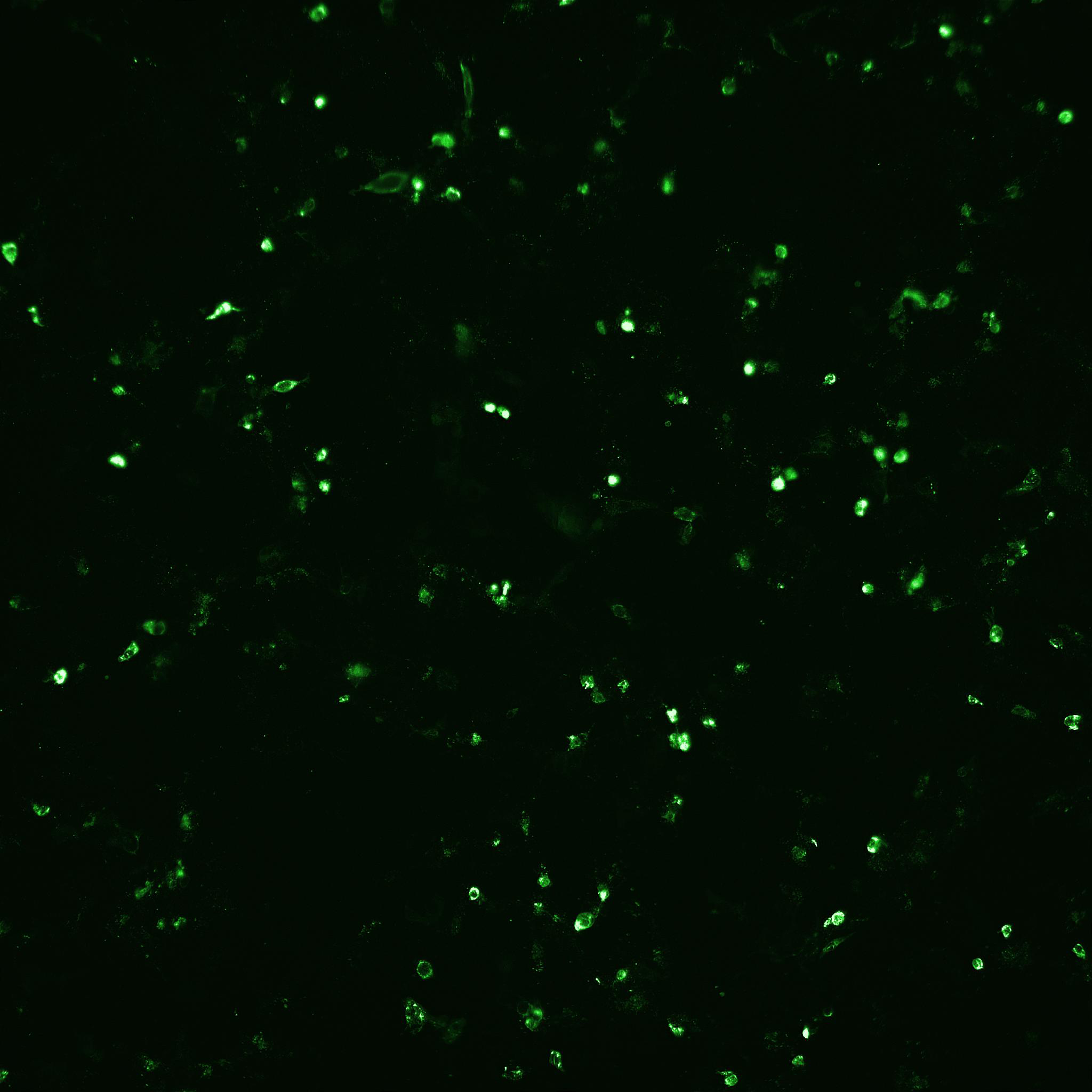
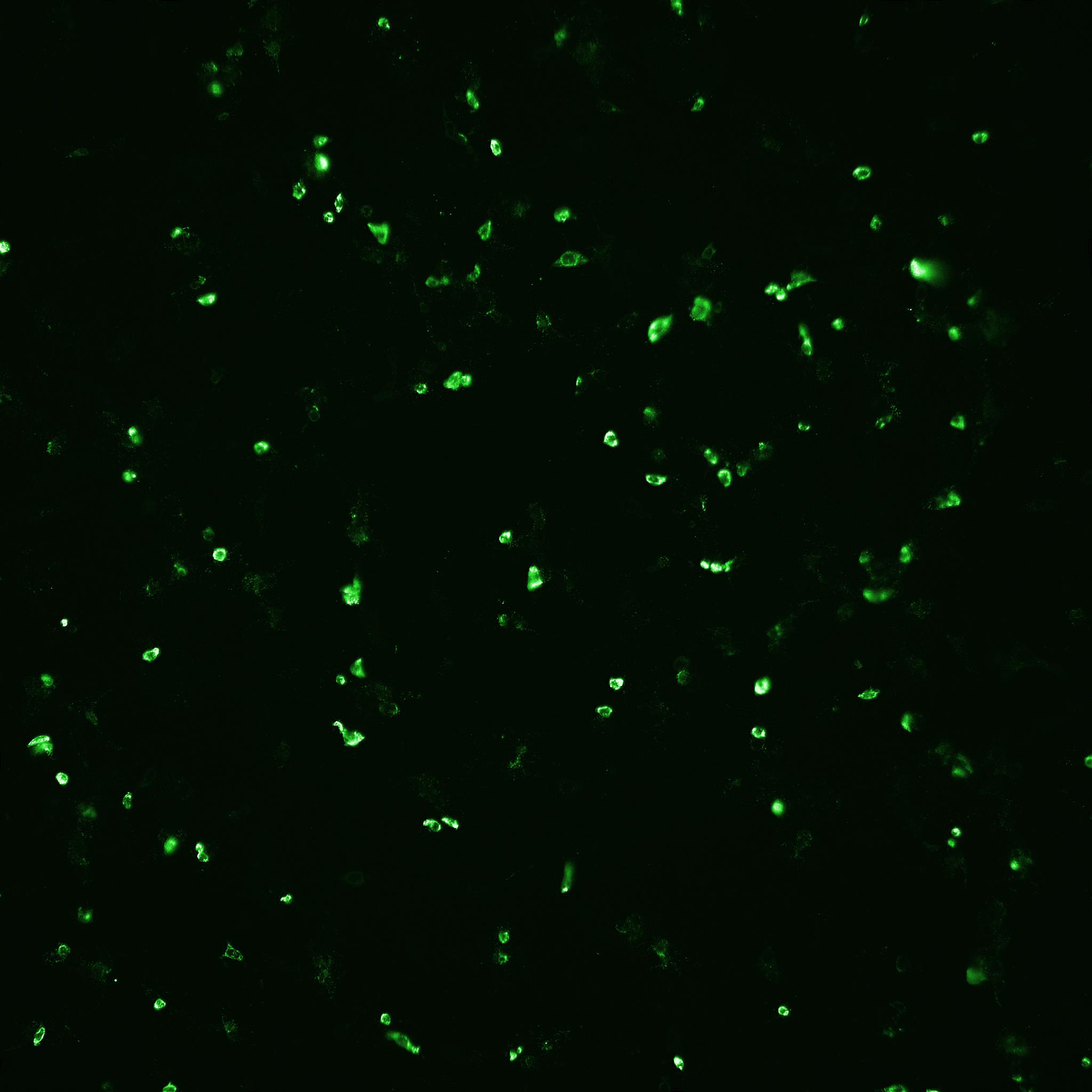
(A) Porcine trypsin
(B) Sheffield rTrypsin ACF
Fig 5. The number of infected cells as well as total number of cells after rotavirus infection mediated with (A) porcine trypsin or (B) Sheffield rTrypsin ACF, were detected and counted by automated fluorescence microscopy using an IN Cell Analyzer 2200.
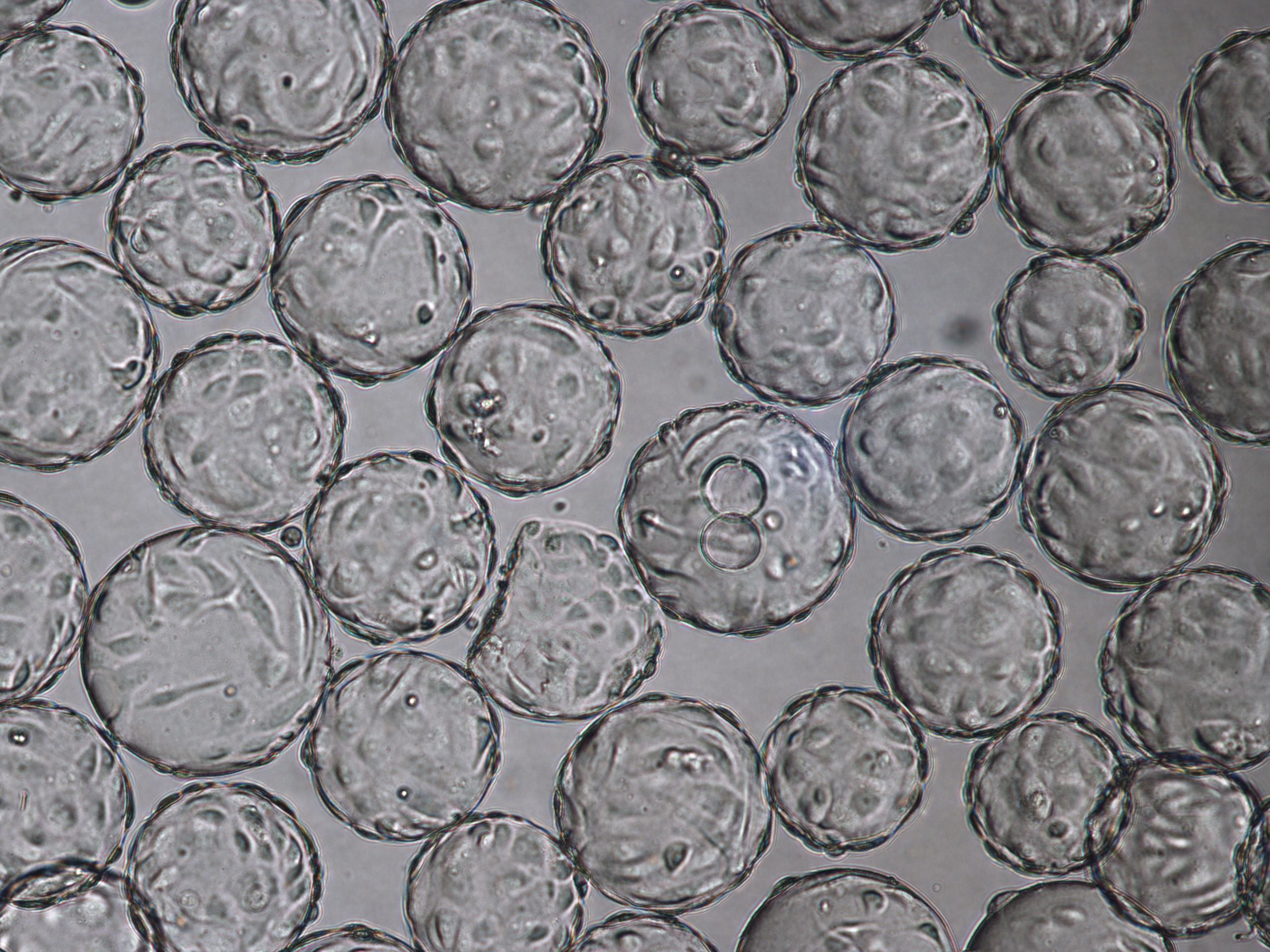
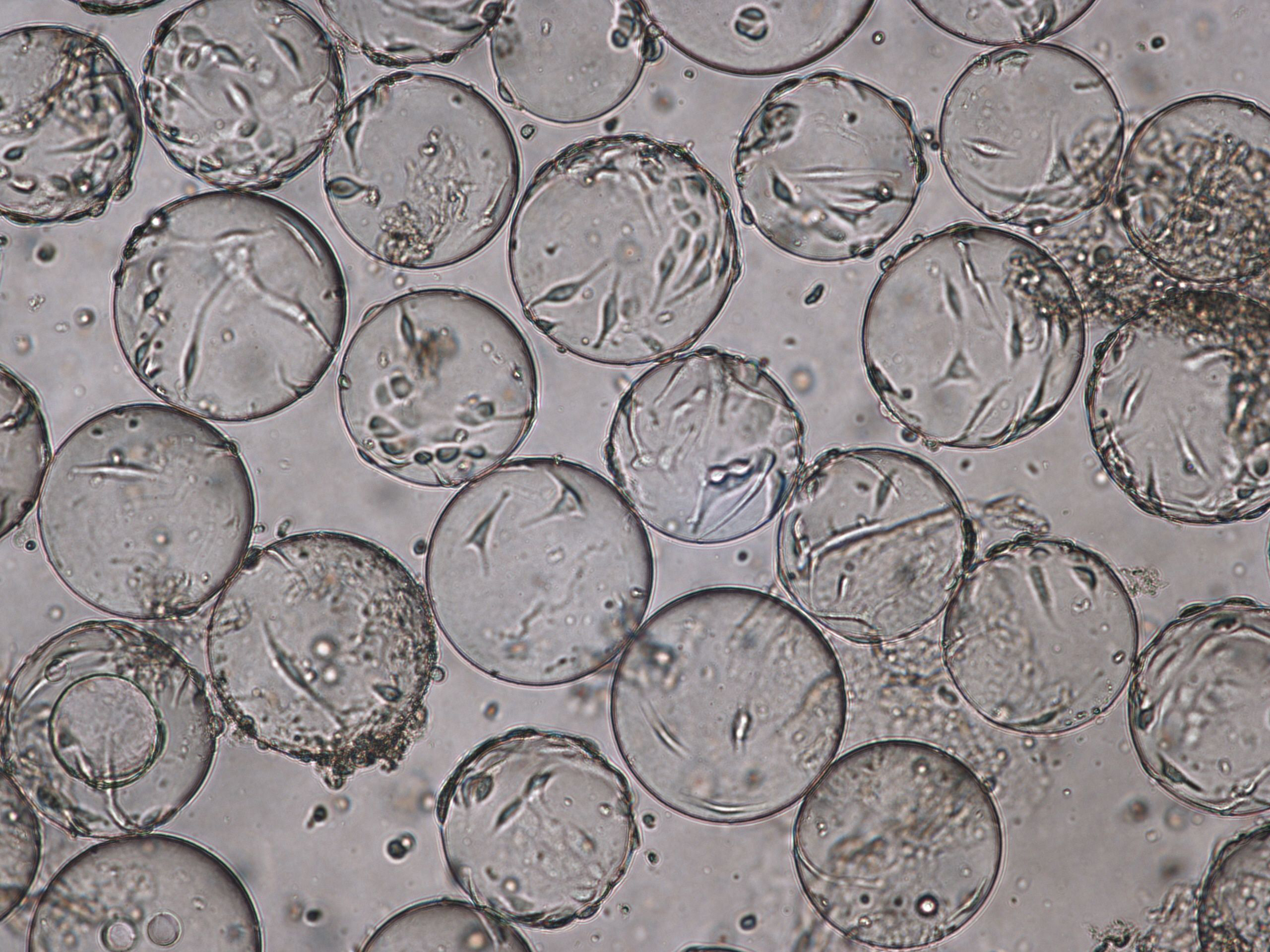
Following the positive results from small-scale culture, we investigated the scalability of the process for rotavirus production. Cell growth and morphology were monitored daily and the results from TOI and TOH are shown (Fig 6).
(A) 96-h cultivation (TOI)
(B) Cytopathic effects for Rotavirus at TOH, 96 hpi.
Fig 6. Cell attachment and morphology of Vero cells on Cytodex 1 Gamma microcarriers using VaccineXpress cell culture medium in a WAVE 25 bioreactor system at (A) TOI and (B) TOH, displaying cytopathic effects from rotavirus propagation.
Rotavirus titer was measured using two methods. ELISA was used to determine total virus. Fluorescence microscopy was used for determining infectious titer (Fig 7 and comparison in Table 3). The bioreactor cultures produced rotavirus titers comparable to the small-scale experiments (compare Tables 2 and 3).
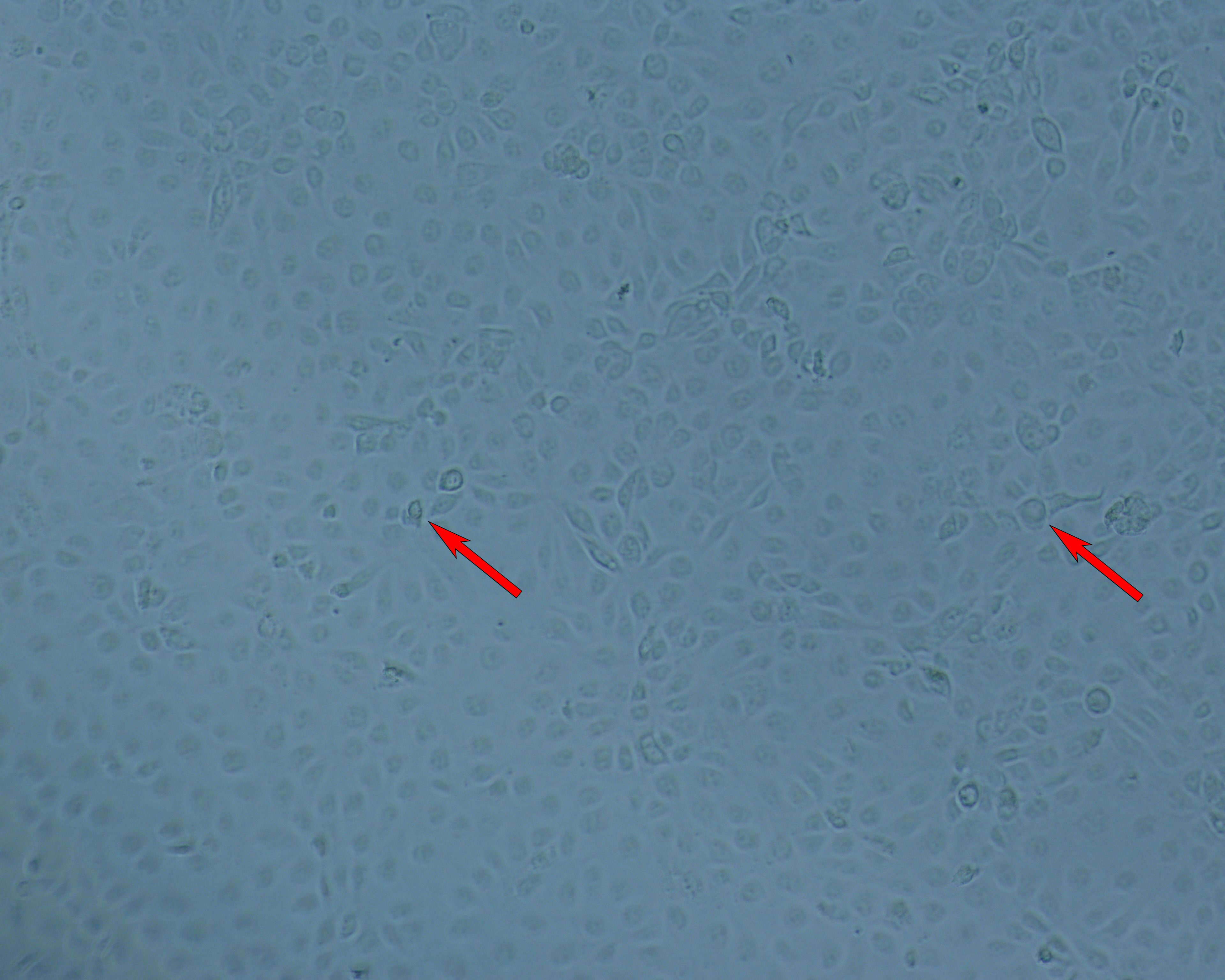
(A) Cell layer; Bright-field.
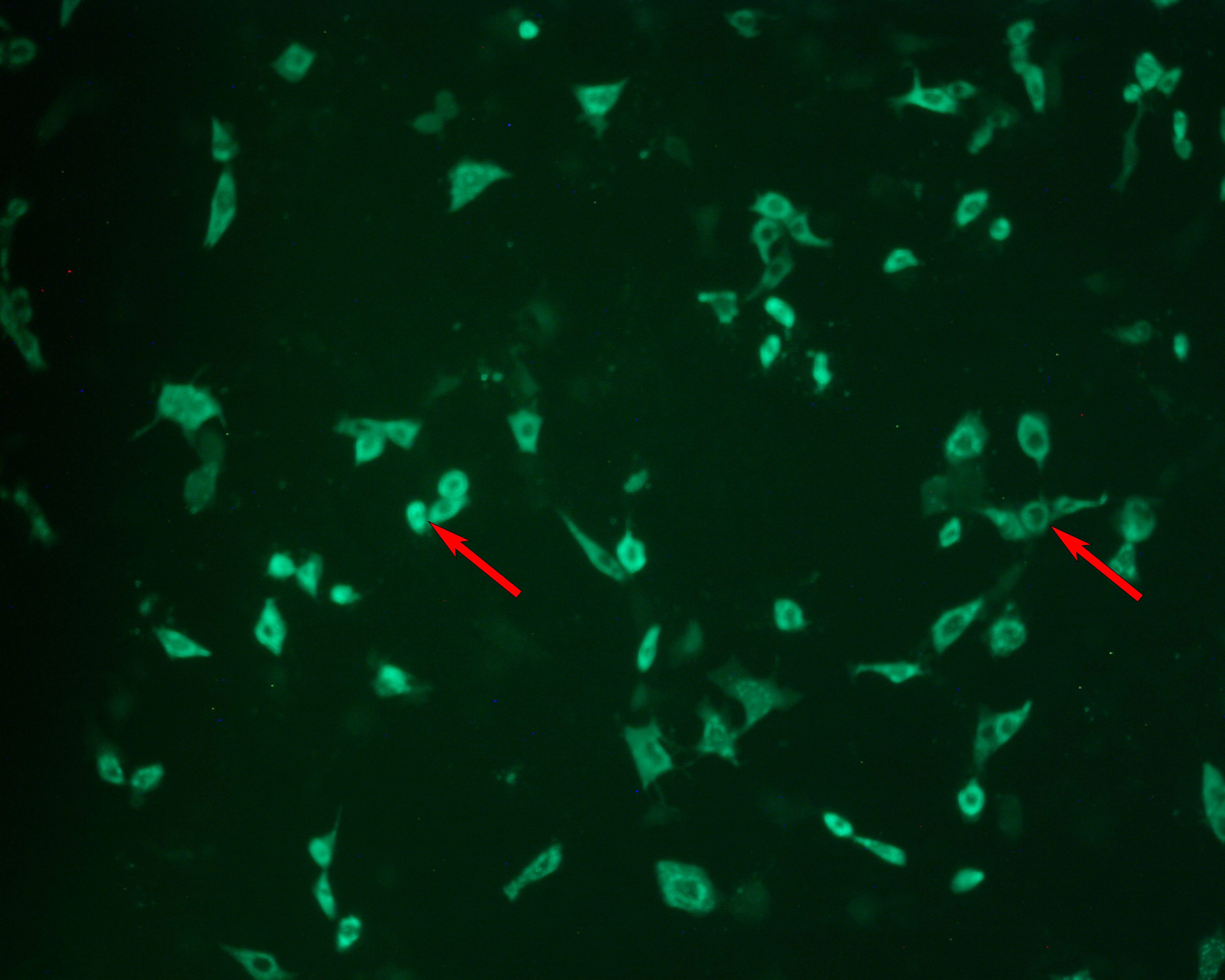
(B) Cell layer; FITC-labeled rotavirus detection.
Fig 7. Focus forming assay (FFA) for measurement of rotavirus infection. (A) Infected cell layer viewed under bright-field microscopy. (B) The same field viewed under fluoresence microscopy. Infected cells are detected using a FITC-labeled antibody raised against rotavirus. Arrows are for points of reference.
Table 3. FFA and ELISA analysis results
| Sample | FFU/mL | ELISA (OD450) |
| Rotavirus | 5.1 × 105 | 1.2 |
| Rotavirus ref sample | 2.6 × 105 | 1.2 |
Conclusion
HyClone VaccineXpress cell culture medium promotes the production of infective influenza virus and rotavirus in a scalable ADCF process. Results from a 2 L working volume in a ReadyToProcess WAVE 25 bioreactor were comparable to results obtained using spinner flasks. Scale-up results from influenza virus production were applied to the propagation of rotavirus. The combination of VaccineXpress medium, Cytodex 1 Gamma microcarriers, and recombinant trypsin formed a novel, ADCF process for the scalable production of infectious rotavirus. This process has the potential to be scaled for rotavirus GMP manufacturing.
Reference
- Reed, L. J. and Muench, H. A simple method of estimating fifty percent endpoints. Am J Epidemiol 27(3), 493-497 (1938).
| Product name |
Product code |
Pack size |
| VaccineXpress cell culture medium | SH31126.01 | 1000 mL |
| L-Glutamine 200 mM | SH30034.01 | 100 mL |
| ReadyToProcess WAVE 25 Rocker | 28988000 | 1 |
| ReadyToProcess CBCU Controller unit | 29044081 | 1 |
| Cellbag 10 L Bioreactor Container with Fortem Film | CB0010L722-31-05PK | 5/bags |
| Cytodex 1 Gamma | 17548701 | 30 g |
| IN Cell Analyzer 2500 HS imaging system | 29240356 | 1 |