We summarize the results of Escherichia coli growth performance for plasmid production in a 5 L glass fermentor, which was scaled up to 80 and 160 L scale in Xcellerex™ XDR-200 MO single-use fermentors. We used an E. coli DH5α strain which produced a pLP2 plasmid of 4180 bp in the study. Performance of peak E. coli growth measured as optical density at 600 nm (OD600), plasmid titer, and percentage of produced supercoiled (SC) plasmid DNA was comparable at all culture volumes tested.
The automated presetting Setpoint Table feature of the XDR-200 MO software allowed automated feed control. Lookup tables of Control enabled full automation of DO through cascade control of agitation, air flow and O2 flow. Further design features of the XDR-200 MO such as impeller design (upper pitched impeller plus lower Rushton impeller) contributed to efficient scale-up to the 160 L scale in XDR-200 MO. Our results highlight the suitability of XDR-200 MO bioreactor in upstream bioprocessing of E. coli, which should be beneficial to facilities producing plasmids for, for example, gene therapeutic applications.
Introduction
Gene therapeutics as well as mRNA and DNA vaccines are manufactured today through use of plasmid DNA, which is produced by E. coli grown through upstream bioprocessing in fermentors. Typically, plasmid production supporting viral vector and mRNA manufacturing only needs a few hundred liters at the most, making single-use solutions very attractive.
Previously, we have used XDR-50 MO bioreactor for several plasmids and microbial production of VLP proteins production, obtaining high optical density (OD) at 600 nm (OD600). Key features of the XDR-50 MO bioreactor contributing to improved E. coli growth are the special impeller design (upper pitched impeller plus lower Rushton impeller), high VVM gas flow, and baffles. Also, the single-use capability gives faster turnaround times and reduces cross-contamination risk.
The single-use XDR-200 MO* comprises the same design features as the XDR-50 MO fermentor. The goal of this study is to determine if the 160 L scale of the XDR-200 MO fermentor is suited to viral vector applications and if the requirements for mRNA/viral vector processes are met.
We show by proof of concept that the performance of a 5 L glass fermentor in terms of E. coli growth and expression of SC plasmid DNA can be reproduced at larger scale in the XDR-200.
For detailed Materials and methods, see the end of this document.
* XDR 200-MO is also available in a dual purpose (DP) configuration where it can be used in cell culture and microbial applications.
Results and discussion
E. coli culture process development: 5 L glass fermentor
The 5 L glass fermentor was inoculated with OD600 of 0.02, and the initial culture volume was 2.5 L. At 8.7 h of growth after inoculation, the DO value decreased to its setpoint (30%), then the air and O2 flow were automatically supplied to the fermentor with the pre-setting cascade control. The feeding rate at start was set to 0.2 mL/min and was increased manually by 10% to 15% every 2 h to maintain the bacterial specific growth rate (µ) at 0.06 to 0.08 h-1.
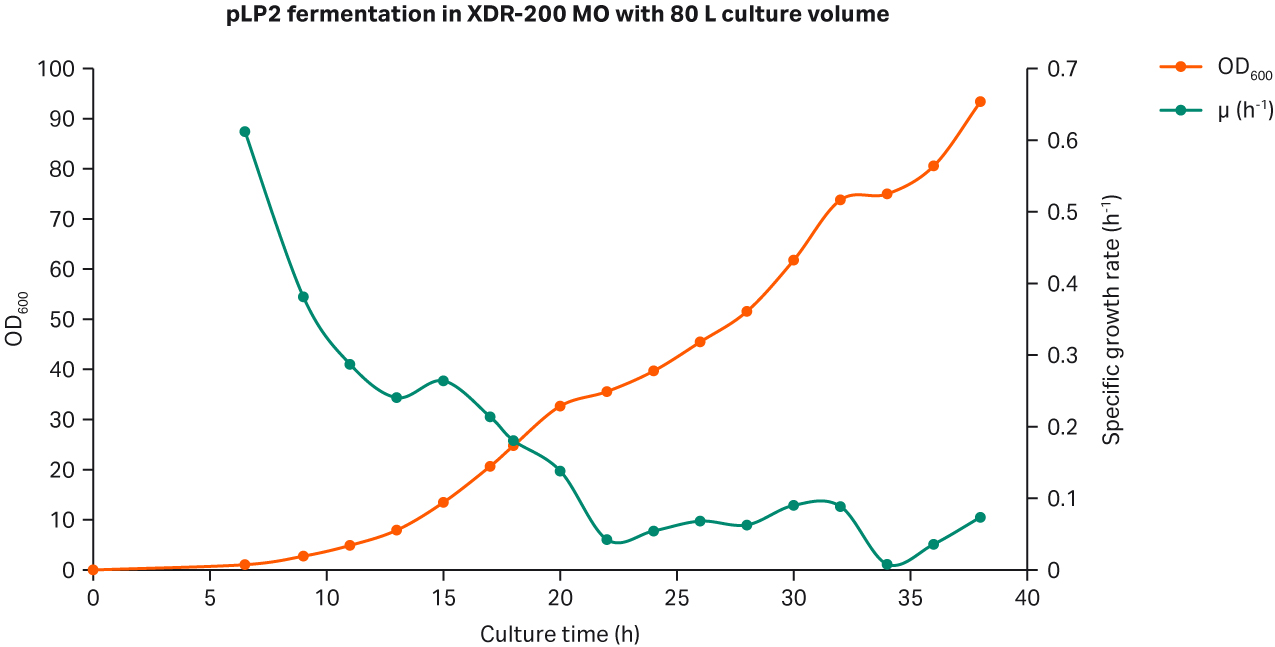
The growth curves are shown in Figure 1. The peak OD600 reached 89.2 at the end of culture process.
(A)
(B)
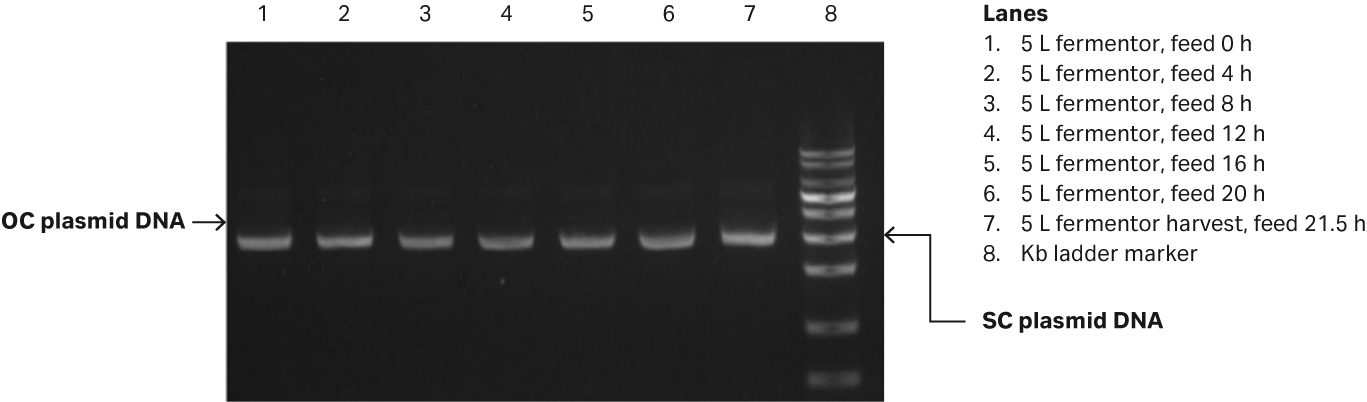
Fig 1. (A) E. coli growth measured in OD600 and specific growth rate/h from the 5 L glass fermentor. (B) Agarose gel electrophoresis analysis of plasmid produced in the 5 L glass fermentor.
Samples were taken every 4 h and frozen at -20°C. After thawing at the end of the fermentation process, the samples were diluted to between 1.0 and 1.5 OD, and the plasmid titer and supercoiled (SC) plasmid percentage were analyzed by spectrophotometry and agarose gel electrophoresis (Fig 1B). In the gel, the SC plasmid migrates as 3000 bp marker, while open circular (OC) plasmid migrates as a 4000 bp marker.
The pLP2 plasmid titer achieved reached 61.5 mg/L, and the SC percentage was 92.2%.
E. coli culture process scaleup in XDR-200 MO fermentor: 80 L culture volume
Scale up to XDR-200 MO fermentor was based on keeping similar volume of gas per volume of liquid/min (VVM) as in the 5 L bioreactor, and the peak VVM was controlled at 0.2 to 0.3. We inoculated the fermentor at OD600 of 0.02 and the initial culture volume was 68 L. The DO setpoint was 30% and cascaded to air and O2 flow. When the O2 gas flow rate reached 9 to 10 Lpm, we increased agitation speed by manually 15 rpm increments to maintain DO.
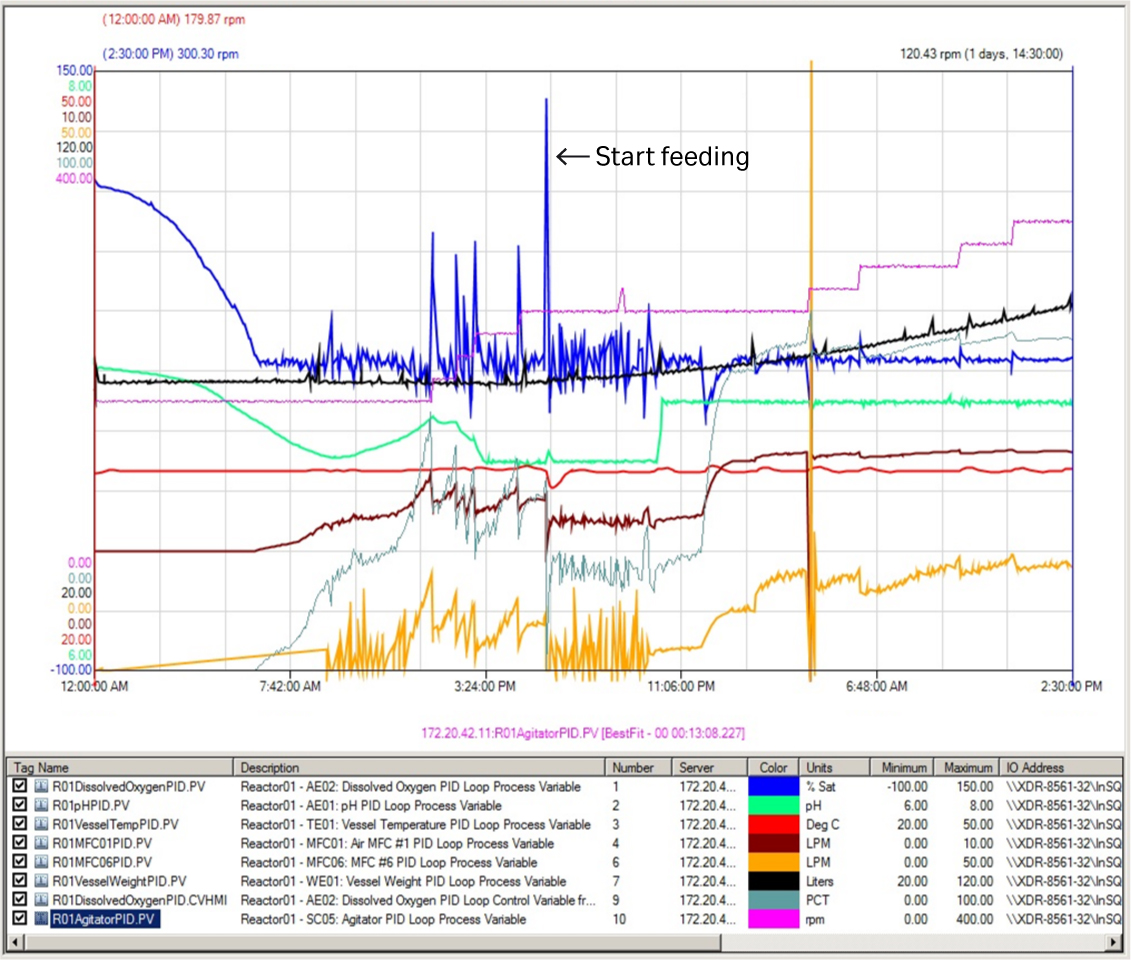
After 6.5 h of fermentation, the DO value decreased for the first time to the setpoint (30%). At this point, the air and O2 gas flow were automatically supplied to the fermentor with the preset cascade control. At 18 h of fermentation, the DO value suddenly spiked to 115%. This was accompanied by a significant reduction in air and O2 gas flow rate indicating depletion of nutrients. Feeding was started at this point at 5.6 mL/min (Fig 2) and was increased manually by 10% to 15% every 2 h to keep the specific growth rate at 0.06 to 0.08 h-1 (Fig 3A).
Fig 2. Key process parameter curves of first XDR 200 MO fermentor run at 80 L culture volume. Orange curve = oxygen; purple curve = manual agitation.
(A)
(B)
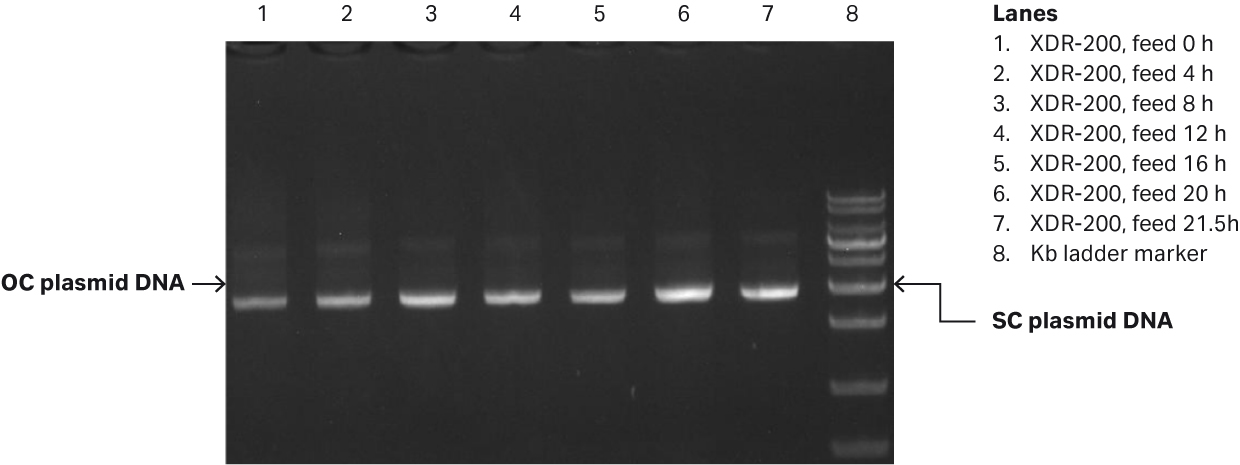
Fig 3. (A) E. coli growth curves in XDR-200 MO with 80 L culture volume. (B) Agarose gel electrophoresis analysis of plasmid produced in the fermentor.
Our analyses revealed that after fermentation process was complete, the plasmid titer was 58.79 mg/L and the SC percentage was 91.49% as determined by gel electrophoresis (Fig 3B, lane 7).
E. coli process scale-up in XDR-200 MO fermentor: 160 L culture volume
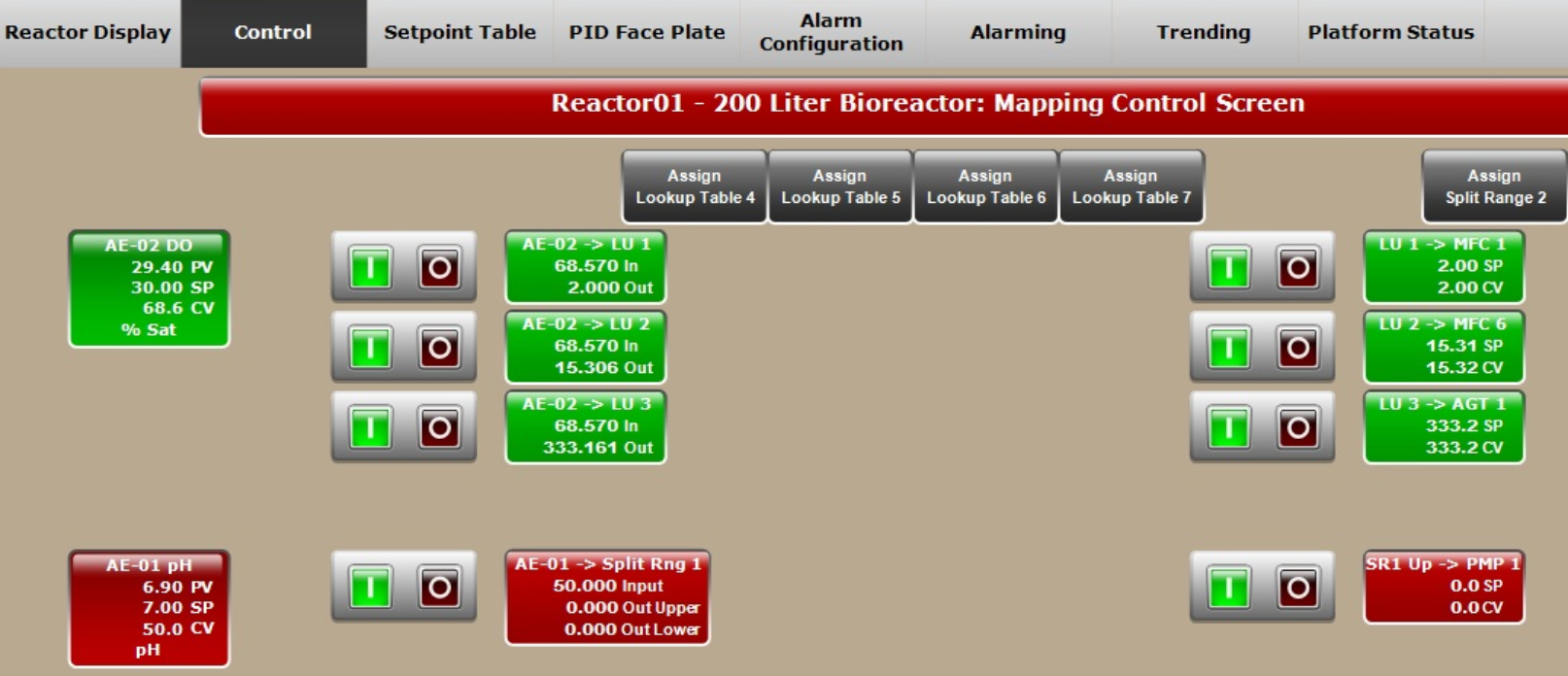
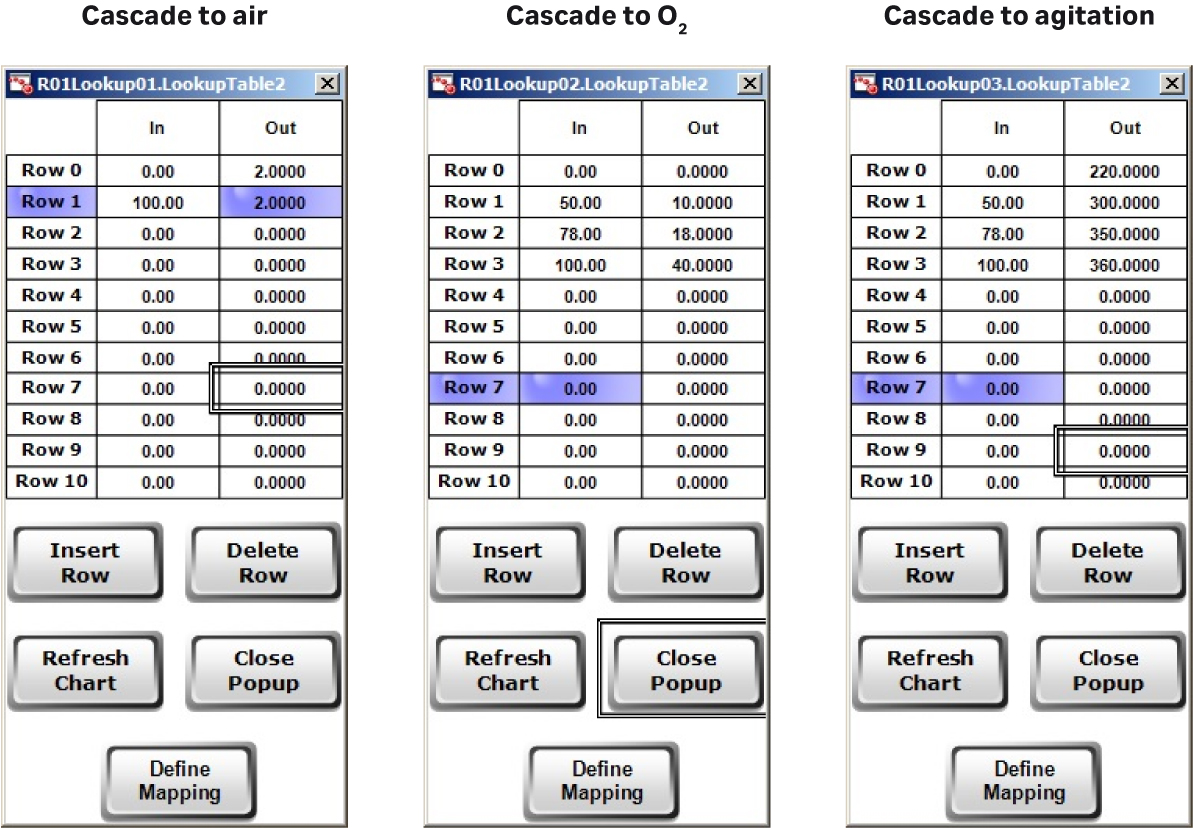
Here, the whole process — including agitation speed and feed rate — was automated compared to the previous runs. XDR-200 MO fermentor employs advanced DO control mode. In the DO control loop, you can customize different DO CV (controller variable) corresponding to different air, O2 flow rate, and agitation rate (Fig 4A), which is advantageous for gas and agitation control strategies. The customized setting not only helps to achieve stable DO control, but also helps to avoid extensive foaming and to control bag pressure.
In this culture batch, the DO was fully automatically controlled and cascaded to air, O2 flow rate, and agitation speed (Fig 4B) using Lookup tables.
(A)
(B)
(C)
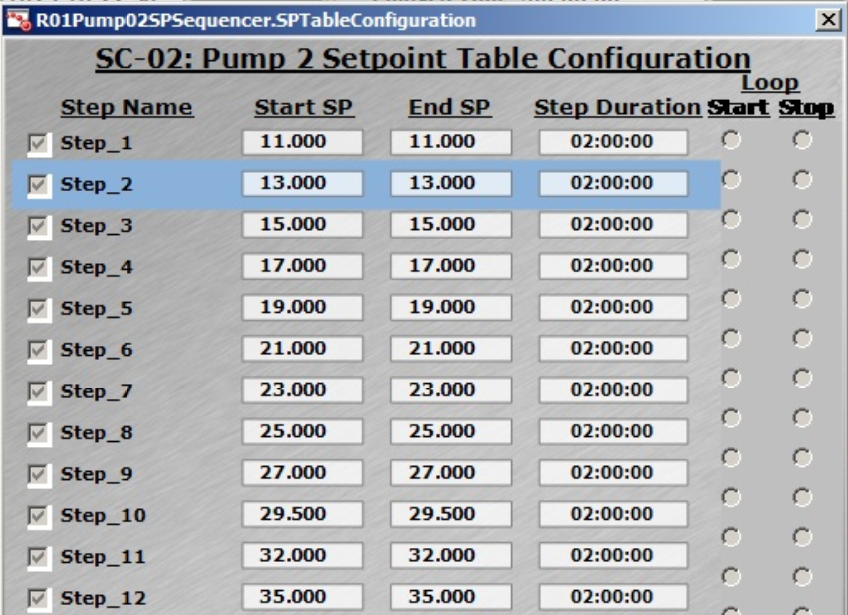
Fig 4. (A) Flexible control strategies of DO setting can be inputted by users. (B) DO control cascading map to MFC1 (air), MFC6 (O2), and AGT1 (agitation speed). (C) Feed rate presetting in Setpoint Table for XDR-200 MO, 160 L culture run.
Feeding rate can be automatically adjusted through the presetting in Setpoint Table (Fig 4C), which simplifies the operation. All the data of feeding rate and feeding amount are recorded in the control software, which reduces the risk of operational errors and better meets regulatory demands.
In this experiment, we performed automatic control of DO and feeding through Control and Setpoint Table setting of the software (Fig 4B and 4C). When the carbon source (nutrient) in basic medium was depleted, the feeding process started, and feed medium was automatically added to the fermentor.
The Setpoint Table setting is shown in Figure 4C — the max. feeding rate in this run reached 35 mL/min (Step_12).
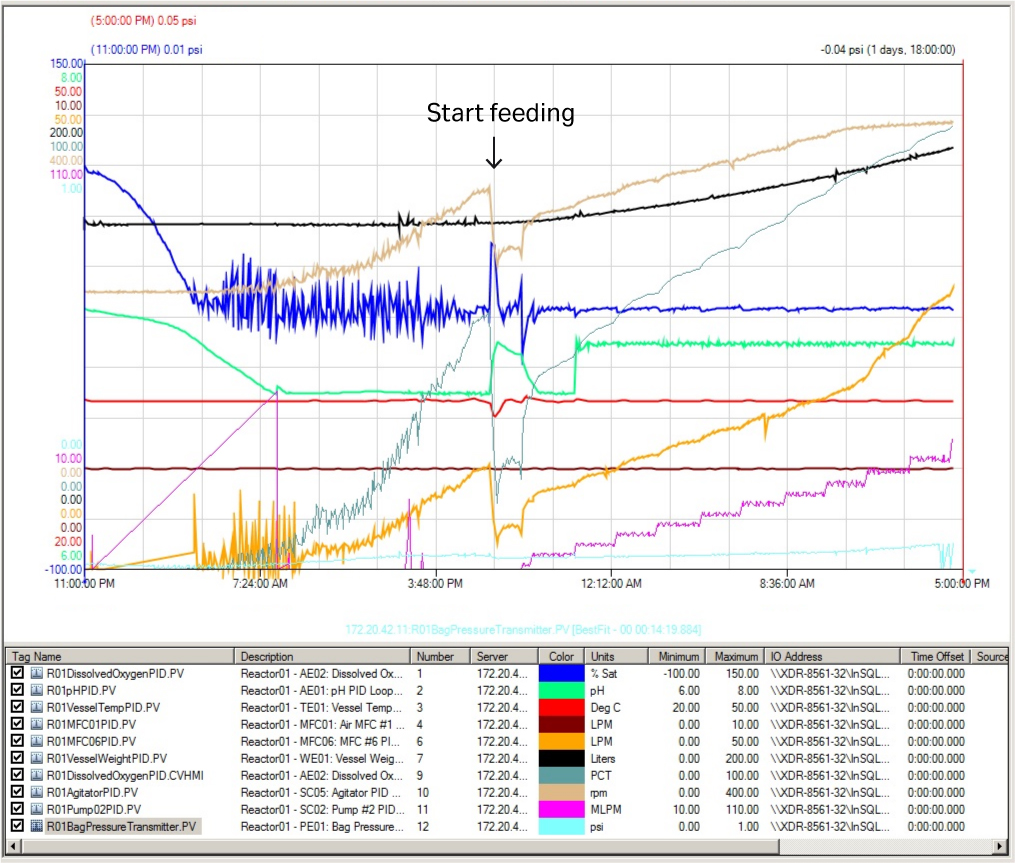
The fermentor key running parameter curves are shown in Figure 5. The key parameters — DO (blue line), pH (green line), and temperature (red line) — were stable with only minor fluctuations over the whole culture process. This shows the excellent control possible with the XDR-200 MO fermentor for all key parameters at this culture volume.
Fig 5. Key running parameter curves in XDR-200 MO fermentor at 160 L culture volume.
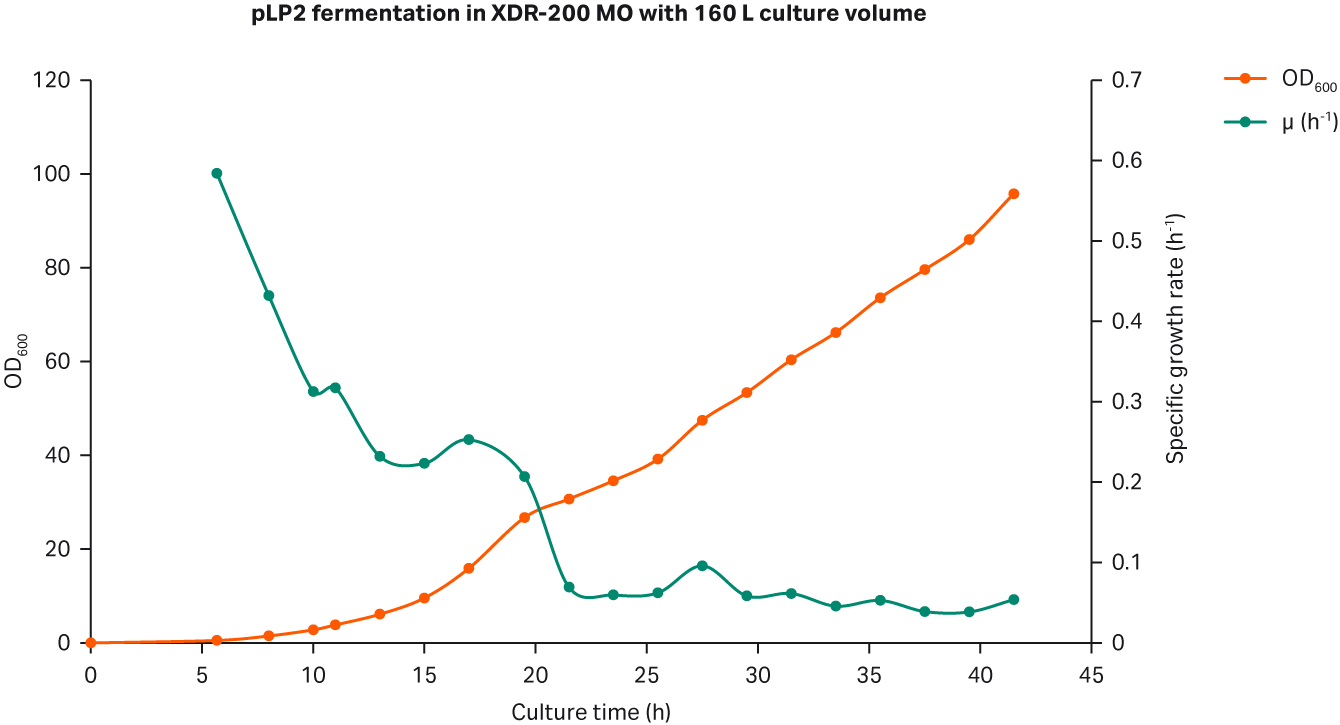
The weight at harvest point reached 167 kg while the peak OD600 reached 95.8. Again, the average growth rate after feeding was maintained at specific growth rate of 0.06 to 0.08 h-1 (Fig 6A).
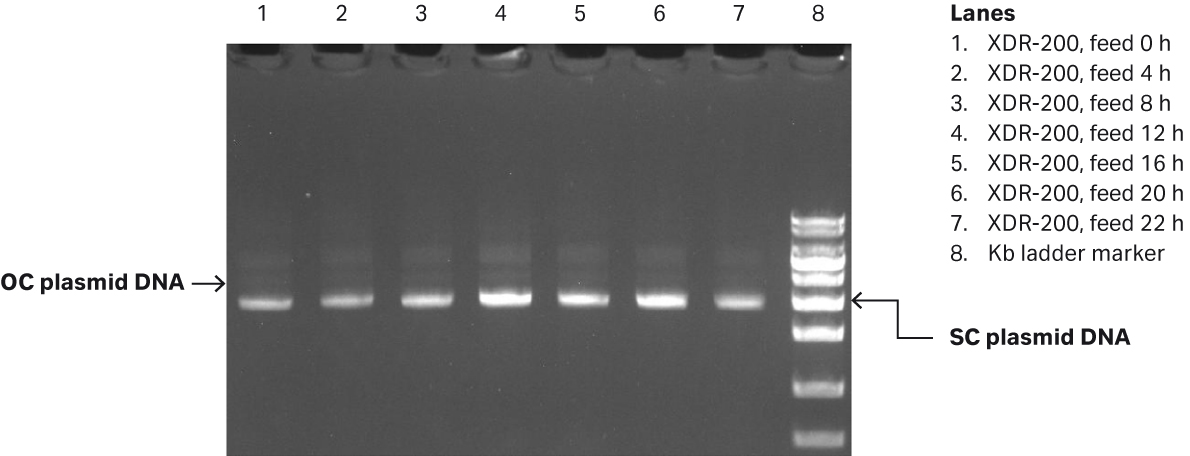
We determined the plasmid titer at 50.6 mg/L while SC percentage was 86.6% as determined by gel electrophoresis (Fig 6B).
(A)
(B)
Fig 6. (A) E. coli growth curves in XDR200 MO with 160 L culture volume. (B) Agarose gel electrophoresis analysis of plasmid produced in XDR-200 MO fermentor.
Summary of E. coli growth, plasmid titer, and SC percentage in 5 L and XDR-200 MO fermentors
The peak OD600 in the 5 L glass and in XDR-200 MO fermentor with 80 and 160 L culture volumes was 89.2, 93.4, and 95.8, respectively. The plasmid titer and SC percentage are summarized in Table 1.
Table 1. Plasmid titer and SC percentage from the 5 L fermentor and 80 and 160 L volume runs in XDR-200 MO
| Lane # | Description | Plasmid titer(mg/L) | SC percentage (GIS method) | ||||
| 5 L batch | 80 L batch | 160 L batch | 5 L batch | 80 L batch | 160 L batch | ||
| 1 | Feed 0 h | 23.997 | 21.888 | 14.503 | 91.76% | 80.36% | 87.72% |
| 2 | Feed 4 h | 26.249 | 29.299 | 25.942 | 90.08% | 83.55% | 88.1% |
| 3 | Feed 8 h | 30.010 | 30.777 | 36.602 | 92.99% | 92.46% | 87.75% |
| 4 | Feed 12 h | 29.940 | 30.578 | 35.984 | 90.56% | 89.09% | 87.46% |
| 5 | Feed 16 h | 46.678 | 51.576 | 44.295 | 93.78% | 87.8% | 89.51% |
| 6 | Feed 20 h | 61.509 | 58.79 | 50.639 | 92.24% | 91.49% | 91.75% |
Conclusions
- Peak OD600 reached 93.4 to 95.8 in XDR-200 MO fermentor, which means that it can support high OD600 E. coli growth.
- Scale up from the 5 L fermentor to 80 and 160 L gave comparable peak OD600, plasmid titer, and SC percentage, which means you can rely on XDR-200 MO fermentor for good E. coli growth and plasmid production.
- We observed no oxygen transfer rate (OTR) issues under the applied specific growth-rate cultivation conditions in combination with high O2 flow relative to air.
Preparation of research cell bank
Ampicillin-resistant E. coli DH5α strain constructed for pLP2 plasmid production was used in this study. The pLP2 plasmid size is 4180 bp with high DNA copy number.
To develop a research cell bank (RCB), we thawed the pLP2 E. coli DH5α strain at room temperature and 100 µL was inoculated in 200 mL of LB medium containing 100 mg/L ampicillin in 1 L shake flasks. After 23 h of growth, the RCB was prepared when OD600 reached 0.898. The RCB with the OD600 of 0.45 and 15% glycerol was frozen and stored at -80°C.
Medium preparation for fermentors
E. coli growth basic and growth feed media were custom-made at Cytiva (Fast Trak China). All components were added into a beaker or in a tank in turn and stirred until all the components were dissolved. We heated the medium to more than 90°C after which it was transferred to SCHOTT-DURAN™ screw-cap glass bottles or Nalgene™ 20 L barrels (Thermo Fisher Scientific) for autoclaving. To ensure thorough sterilization, we kept the loading volume in the 20 L barrel to no more than 10 L.
Plasmid titer and SC percentage testing
During the fermentation process, samples were taken from 5 L or XDR-200 MO (200 L) fermentors every 4 h from feeding point and frozen at -20°C. After finishing batch culture, all the samples from one batch were diluted to OD600 of 1.0 to 1.5 and samples of 6 mL were taken for plasmid extraction. Plasmid extract was loaded to a spectrophotometer for titer evaluation; a total of 2 µL of the plasmid extract (approx. 300 ng of plasmid) was loaded to an agarose gel for electrophoresis. Percentage SC plasmid was determined from the gel using GIS software.
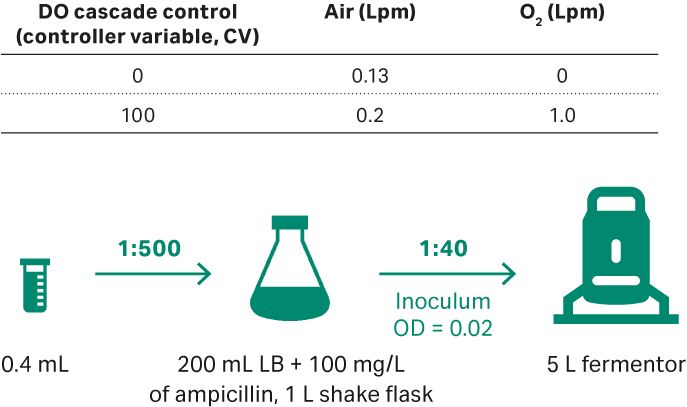
E. coli DH5α culture process development in 5 L glass fermentor (3 L culture volume)
After thawing, E. coli DH5α was seeded in 1 L shake flasks containing LB medium and 100 mg/L ampicillin (Fig 7). Shake conditions were 200 rpm, 30°C and samples were taken for cell density check at OD600. Plasmid titer and SC percentage were evaluated by agarose gel electrophoresis of feed samples at 0, 4, 8, 12, 16, and 20 h.
Fig 7. (A) E. coli upstream culture for plasmid development workflow in 5 L fermentor.
The 5 L fermentor was run with the conditions below:
- Initial inoculum OD = 0.02.
- 2.5 L of ECFT basic medium, and ECFT feed medium.
- 30°C; DO 30%.
- pH 7.00 ± 0.3 at initial culture, and changed to 7.00 ± 0.1 when OD = 30.
- Impeller speed of 300 to 470 rpm, when O2 gas flow rate reached 0.95 to 1.0 Lpm; manual increase by 15 rpm every time.
- OD = 24.4, feeding start at 0.2 mL/min (i.e., 0.08 mL/min/L of culture).
- With the strain growth, increase the feeding rate to maintain the bacterial specific growth rate at 0.06 to 0.08 h-1.
- Culture harvested when the growth OD600 reaches maximum and no longer increases, after which samples are frozen at -20°C.
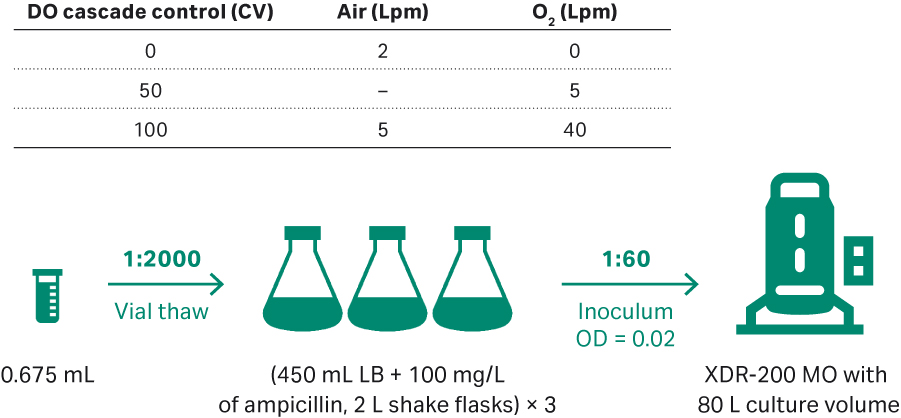
Process development in XDR-200 MO with 80 L culture volume
Seeding for the first run in XDR-200 MO with 80 L culture volume is described in Figure 8. Sampling was performed every 2 h for cell density evaluation at OD600 and gel electrophoresis was performed on harvests taken from feeding time 0 and every 4 h intervals up to the 20 h harvest point.
Fig 8. (A) E. coli upstream culture for plasmid development in an 80 L volume in XDR-200 MO fermentor.
The running conditions for this first run in the XDR-200 MO fermentor were:
- Initial inoculum OD = 0.02.
- 68 L ECFT basic medium as the starting, and ECFT feed medium.
- 30°C; DO 30%.
- pH 7.00 ± 0.3 at initial culture, and changed to 7.00 ± 0.1 when OD = 35.0.
- Impeller speed 180 to 300 rpm when O2 flow rate reaches 9 to 10 Lpm, manual increase 15 rpm every time.
- OD = 24.8, feeding start at 5.6 mL/min.
- Control specific growth rate at 0.06 to 0.08 h-1 by adjusting feeding rate. Feeding rate increases by 10% to 15% every 2 h.
- Culture harvested when the growth goes into plateau and samples frozen at -20°C.
Process development in XDR-200 MO with 160 L culture volume
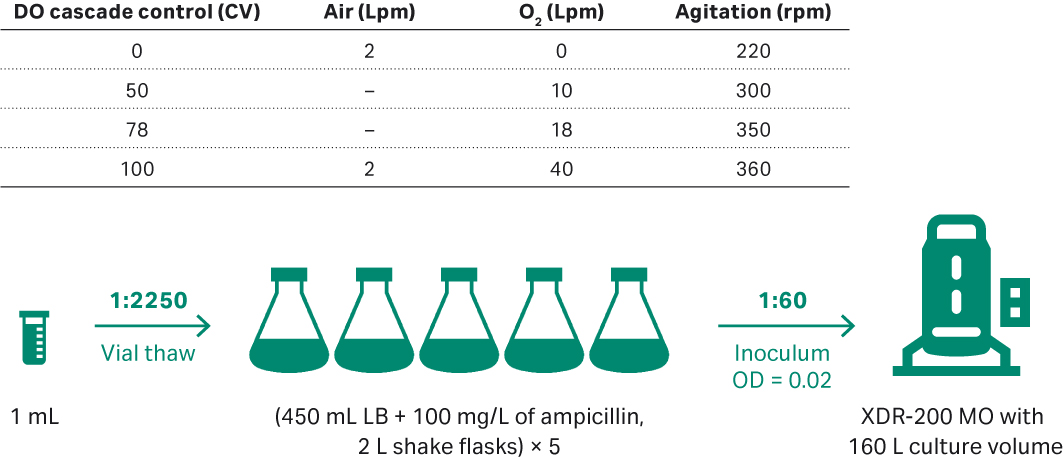
The E. coli from the previous 80 L bioreactor run was scaled up to 160 L according to Figure 9. E. coli DH5α seeds were inoculated in 5 units of 2 L shake flasks containing 450 mL LB medium and 100 mg/L ampicillin. Shake conditions were 200 rpm and samples were taken for cell density evaluation at OD600. When the OD600 reached 1.13, transferred all the seeds to XDR-200 MO fermentor for inoculation. Plasmid titer and SC percentage were evaluated by agarose gel electrophoresis of feed samples at 0, 4, 8, 12, 16, 20, and at harvest point (22 h).
Fig 9. (A) E. coli upstream culture for plasmid development in 160 L volumes performed in XDR-200 MO fermentor.
XDR-200 MO fermentor conditions for this part of the study were:
- Initial inoculum OD = 0.02.
- 136.8 L of basic medium as the starting, and feed medium.
- 30°C; DO 30%.
- pH 7.00 ± 0.3 at initial culture, and changed to 7.00 ± 0.1 when OD = 34.6.
- DO cascade to air, O2, and agitation, and automatic control.
- Max. agitation speed at 355 rpm, and the max O2 gas flow reached 27.7 Lpm in whole process.
- OD=26.7, feeding start at 11 mL/min.
- Automatic feeding using software feature Setpoint Table.
- Culture harvested at 40 to 42 h.
TR29785567
CY24065-26Nov21-AN