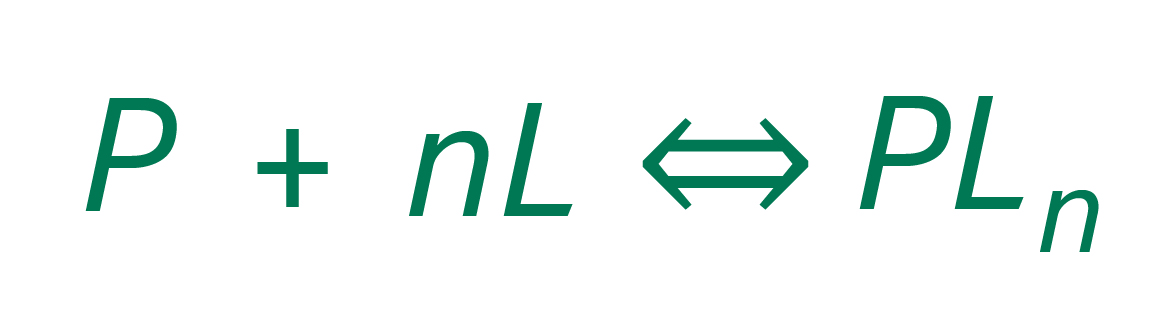In order to create a complete chromatography model, the adsorption of components within the adsorber beads needs to be considered. In this article we will discuss adsorption models for ion exchange, hydrophobic interaction, and mixed mode chromatography.
Prevalent models describe the concentration of adsorbed species as a function of the species’ concentrations in the mobile phase and the concentration bound to the adsorber. In fact, most of the models employed today consider adsorption as a reaction, where one mol of proteins (P) and one mol of ligands (L) react to one mol of a protein-ligand complex (PL). In the case of large proteins, multi-point binding often occurs so that the needed number of ligands is increased by a stoichiometric factor of n.
In the next step, the law of mass action is applied to this chemical equation. It states that the molar concentrations of the reactants taken to the power of their stoichiometric factors is equal to an equilibrium constant K. This results in the equilibrium isotherm equation, a mathematical model that can be coupled directly to the equation system describing fluid dynamics.
Usually, both adsorption and desorption are very fast processes. In many models, the instantaneous formation of the equilibrium is assumed. In general, the isotherm equation is expressed by the rate of change of the concentration of the adsorbed species over time.
Langmuir Model
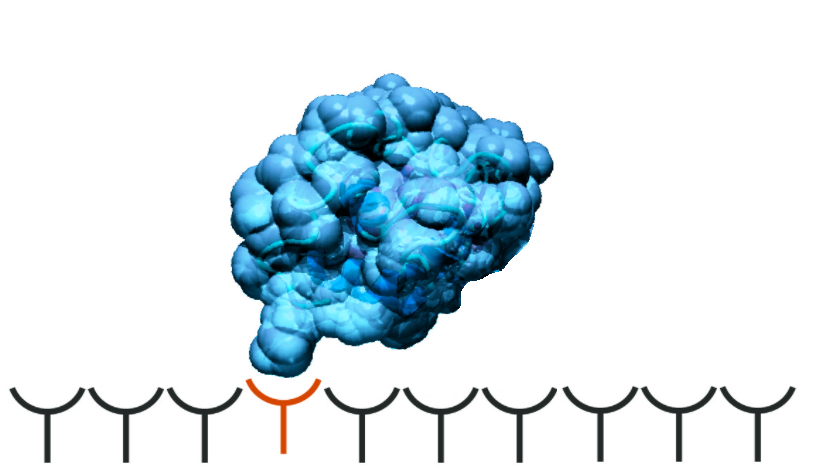
Historically, the Langmuir isotherm is the most extensively studied model. This model considers single-point binding only. It is isocratic, which means the mobile phase does not change its composition during a run. A change in the buffer concentration will not have any effect on adsorption. Due to lacking practical relevance in preparative chromatography, this model in its basic form is predominantly used only in academic research.
Fig 1. Illustration of single-point protein binding.
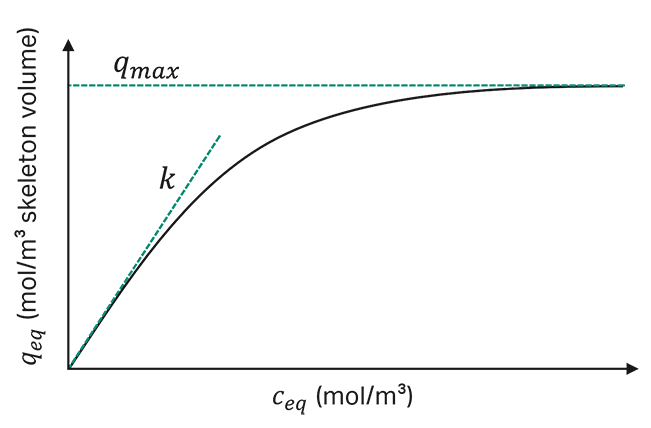
The typical Langmuir-shaped relation between the protein concentration in the stationary phase and the protein concentration in the mobile phase is shown in Figure 2. At low protein concentrations in the mobile phase, the ratio is linear and described with the slope k. At higher loading concentrations, the concentration in the stationary phase reaches a maximum binding capacity called qmax. The ratio between q and c results in the characteristic isotherm curve.
Fig 2. Relationship between protein concentration in the stationary phase and mobile phase for Langmuir model.
Colloidal particle adsorption (CPA) model – ion exchange chromatography
The CPA model for ion exchange chromatography, as developed by and implemented in the GoSilico™ Chromatography Modeling Software, describes protein adsorption using the colloidal nature of proteins. In contrast to the adsorption models discussed below, the CPA model is not based on a simple stoichiometric equation, but on a fundamental description of electrostatic interactions within ion-exchange columns as a function of ionic strength and pH (Fig 3).
The model accounts for steric hindrance at the surface of the adsorber and electrostatic interactions between adsorbed proteins due to increasing protein interaction in the mobile phase. The saturation capacity of the adsorber is thereby physically limited by the surface area accessible to proteins. The more the surface area of the adsorber is covered by adsorbed proteins, the lower the probability of a protein in the mobile phase hitting the adsorber surface.
Fig 3. CPA model for the adsorption of proteins within an ion exchange column as a function of ionic strength and pH.
Steric mass action (SMA) model – ion exchange chromatography
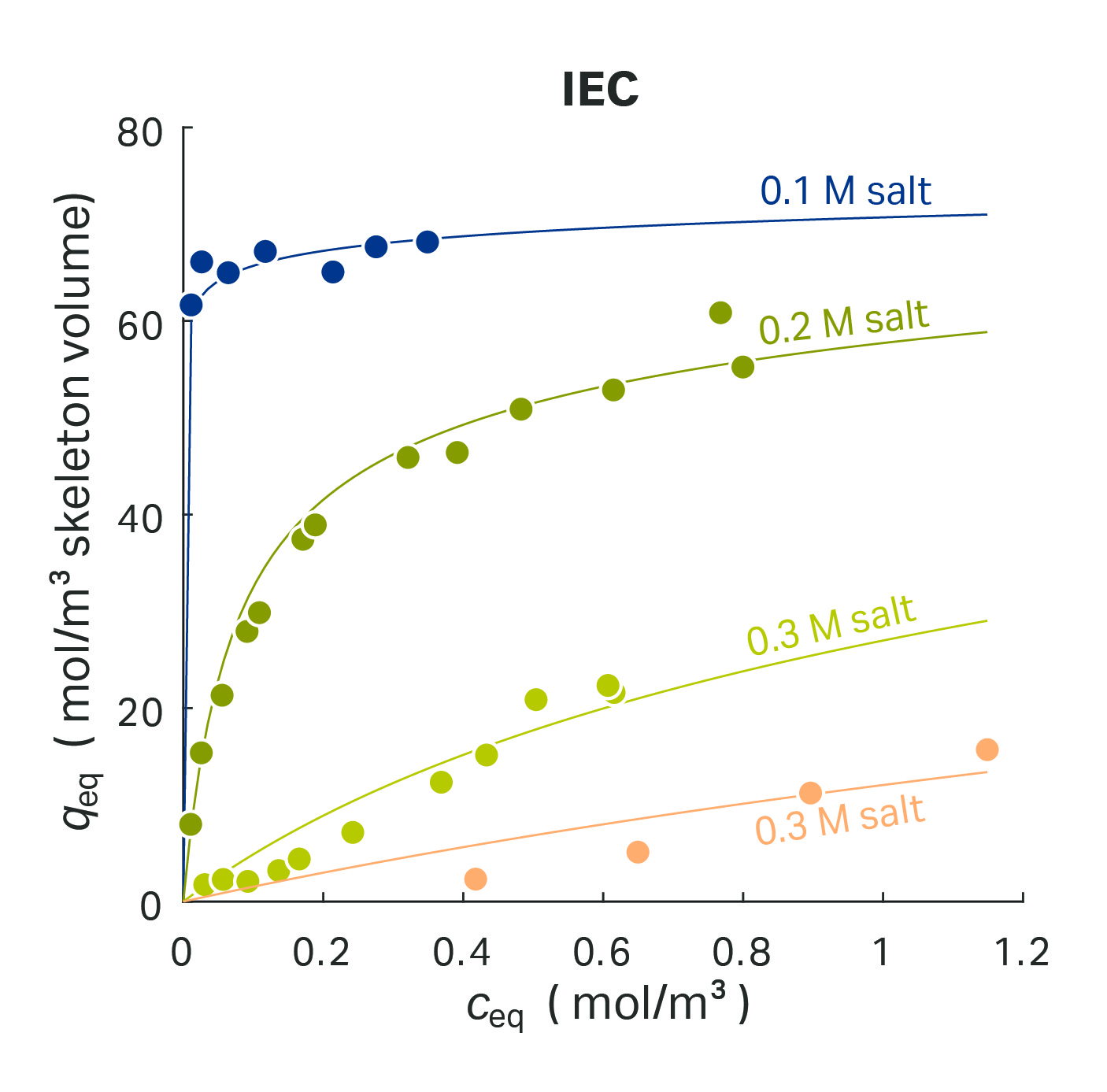
The SMA isotherm is a common model for ion exchange chromatography (IEC) using multi-point binding, the influence of counter-ions, and displacement. This isotherm model is based on a stoichiometric exchange of ions on an oppositely charged surface. The adsorbing protein is assumed to free v moles of salt ions which reside on the ligands involved in binding. The reaction equation becomes:
At constant buffer composition, the SMA model has a very similar shape compared to the Langmuir model (Fig 4). With an increasing mobile phase concentration, a state of saturation is reached. In contrast to Langmuir, this saturation concentration is not a protein-specific qmax but expressed as a function of the total ionic capacity.
Fig 4. SMA model for protein adsorption within an ion-exchange column.
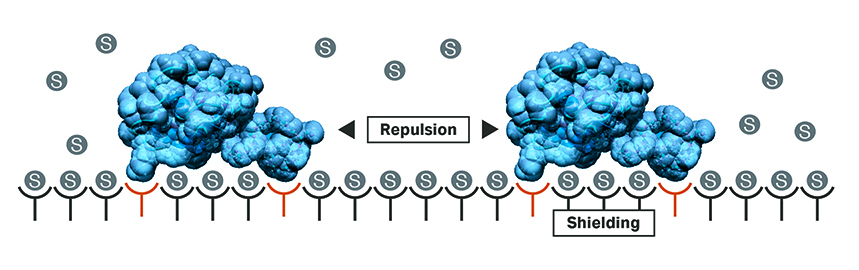
This allows the study of the influence of raw material variability once the model is built. Further effects included in the model are steric hindrance and repulsion. When approaching saturation, a certain number of binding sites cannot be accessed by proteins in the solution anymore. This is because they are sterically shielded or unusable because of stronger repulsive forces (Fig 5).
Fig 5. Illustration of steric hinderance effects of protein adsorption due to repulsion between proteins.
Hydrophobic interaction model
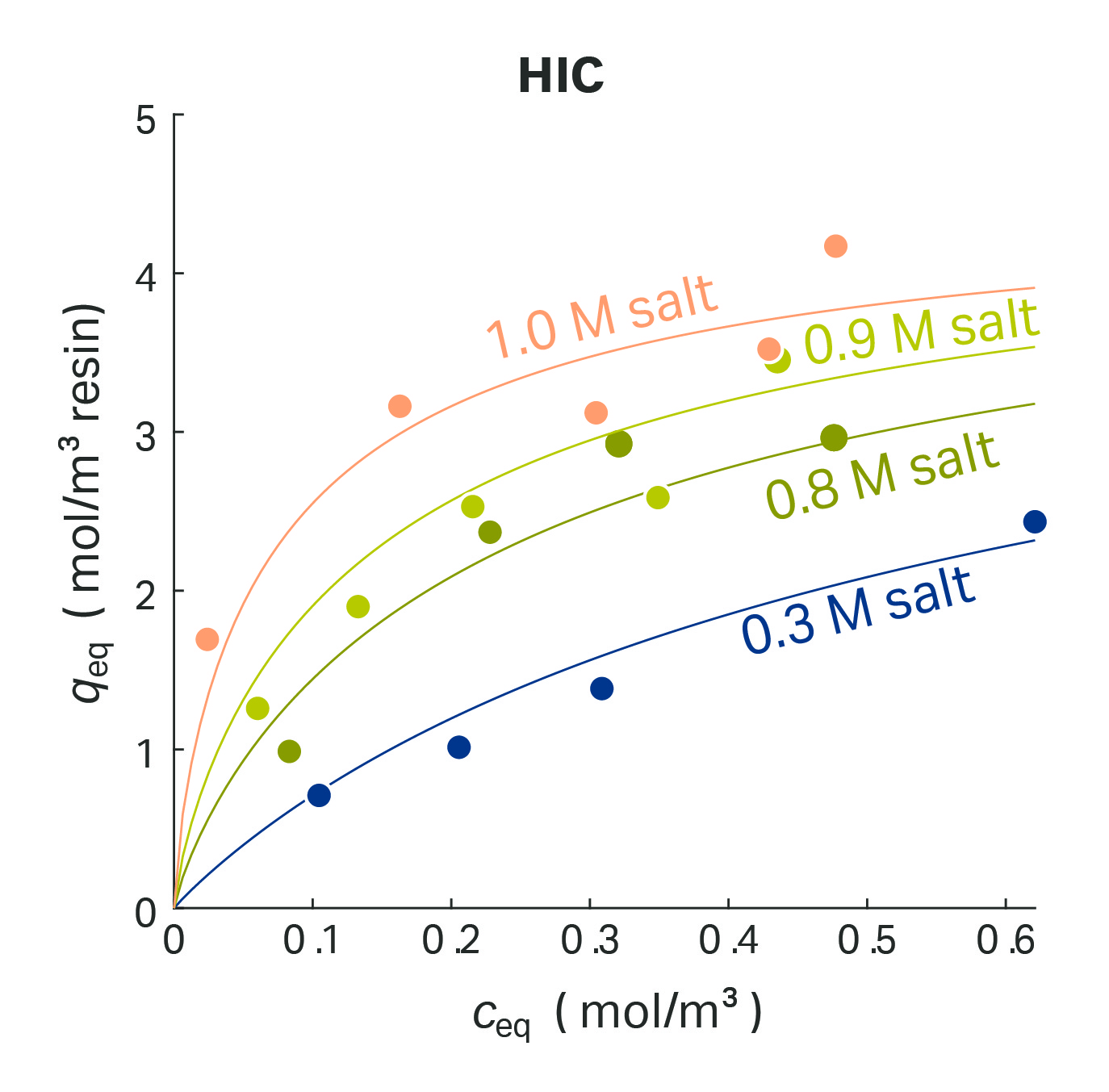
The separation principle of Hydrophobic Interaction Chromatography (HIC) is based on the reversible interaction between hydrophobic patches on the protein surface and the hydrophobic ligands of the stationary phase. Advances in the fundamental understanding of HIC have been achieved by modeling work based on Mollerup’s thermodynamic framework. It extends the simple equations derived from the law of mass actions with so-called asymmetric activity coefficients. This allows the simulation of salt-controlled chromatography processes.
The HIC isotherm as implemented in the GoSilico™ Chromatography Modeling Software considers an equilibrium between well-ordered and bulk-like ordered water molecules on the hydrophobic surface. The challenge of incorporating salt effects in HIC modeling was solved by formulating the hydration number of ions as a function of the salt concentration (Fig 6).
Fig 6. Protein adsorption using the hydrophobic interaction model.
Mixed mode chromatography (MMC)
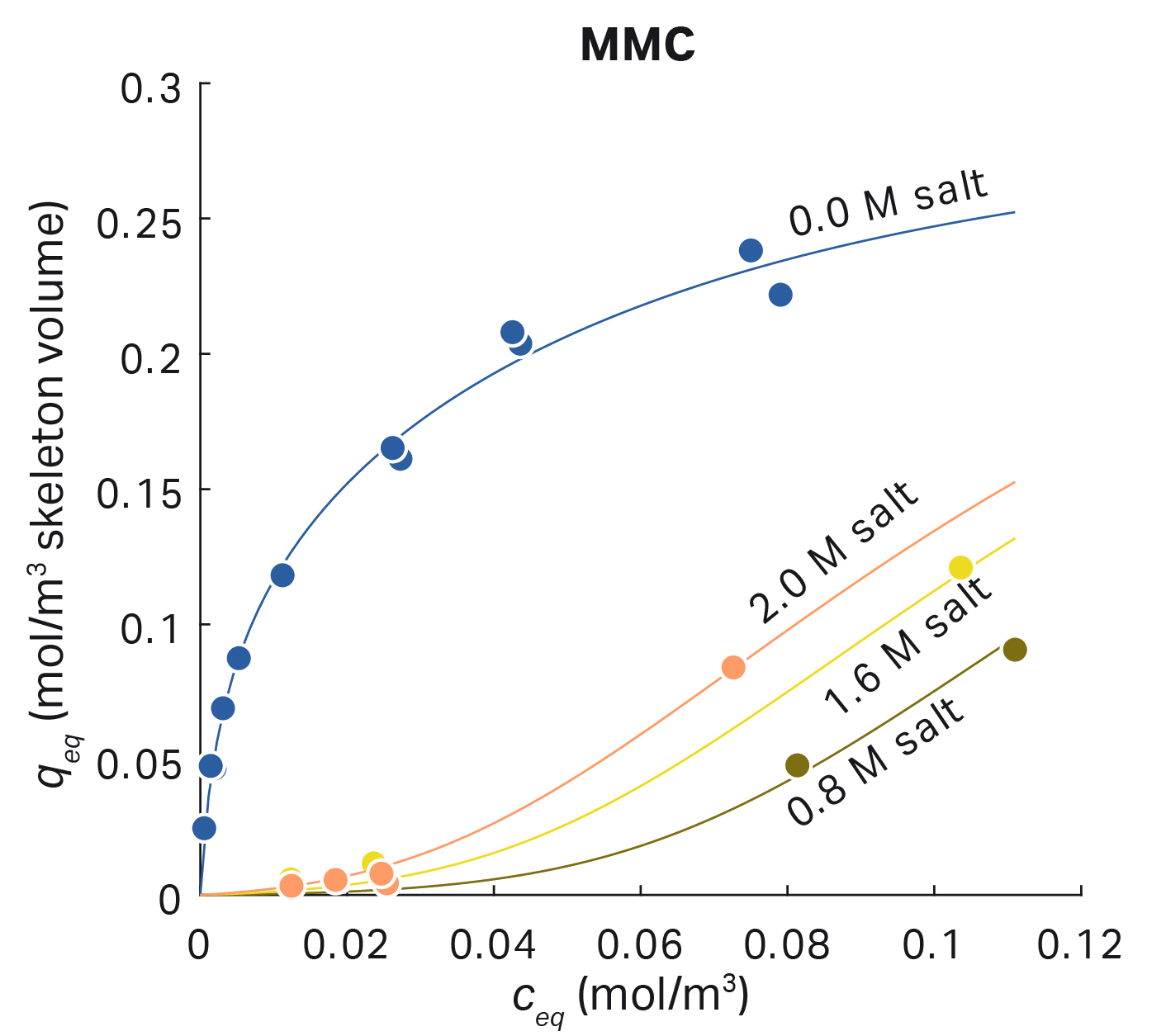
The basic assumption behind current MMC isotherms is that both adsorption modes described above, ion exchange and hydrophobic interaction, take place at the same time. The reaction equation can be extended accordingly so that binding requires both charged and hydrophobic binding sites. The resulting equation is a combination of the Steric Mass Action (SMA) model and Mollerup’s HIC model with asymmetric activity coefficients.
A similar combination of SMA with HIC models within the GoSilico™ Chromatography Modeling Software has produced equally good results using fewer protein-specific parameters. In the industrial use of chromatography, an understanding and model of both interaction modes is rarely needed. Often, mixed mode adsorbers are used as a replacement for process steps for pure ion exchange or hydrophobic interaction that were used in the past. If the process is run in a similar fashion, it is sufficient to assume that one mode dominates and model the MMC as IEC or HIC.
Fig 7. Protein adsorption using the mixed mode chromatography model
Further chromatography modes
Other modes of chromatography which have been successfully modeled in the past are reverse phase chromatography (RPC) and affinity chromatography (AC). Protein A chromatography is of interest, especially for mAb processes. However, the model derivation quickly leaves the path of formulating chemical equations and applying the law of mass action.