Accelerate flavivirus vaccine production with modern tools and solutions
Flavivirus vaccine development and production constitute many challenges and can be both space-and resource-consuming. This article gives an overview of modern tools and solutions, adding flexibility and speed to both upstream and downstream operations in flavivirus vaccine production. Single-use production bioreactors and chromatography purification columns mitigate cross-contamination risk and support increased operator safety, while reducing time to market by eliminating costly and time-consuming cleaning operations. By allowing for quick startup and changeover between productions, single-use technologies provide the flexibility required to quickly adapt to market needs. For downstream processing, modern chromatography resins offer high selectivity and excellent pressure-flow properties for high productivity in manufacturing-scale purifications.
Introduction
As with all viral vaccines, the complex nature of flaviviruses makes process development technically challenging. In addition, vaccine production can be both costly and difficult to scale to meet market demands. In egg-based vaccine production, for example, 100 to 300 vaccine doses can be produced from one fertilized hen egg. However, the eggs used for production need to be supplied from special pathogen-free chicken flocks, limiting availability of eggs and making vaccine production difficult to scale. To meet the need of preventive campaigns, including routine immunization and emergency response stockpiling, millions of vaccine doses would be required, making production both space- and resource-consuming. For a more efficient response to market needs, cell-based vaccine production can be an alternative to egg-based production. However, cell-based vaccine production is traditionally performed in stainless steel bioreactors that require extensive cleaning and sterilization preparation time. Alternative single-use equipment minimizes the need for costly and time-consuming cleaning operations, as manufacturing components that have been in contact with the process material can be disposed after use. Single-use equipment also minimizes cross-contamination risk and contributes to increased operator safety by eliminating the need for open handling of the product. The reduced need for cleaning and cleaning validation allows for quick start-up and changeover between production campaigns. As less cleanroom space is required, single-use technologies help reduce manufacturing footprint as well as costs for utilities, heating, ventilation, and air conditioning.
Cells commonly used for virus propagation, such as Vero cells, are anchorage dependent and can only proliferate when provided a suitable surface. To meet this need in bioreactor cultures, microcarriers are used. Compared with traditional shake flask systems and roller-bottles, microcarriers provide a larger surface area-to-volume ratio, allowing for higher titers at a reduced footprint.
Increasing upstream titers, however, puts pressure on capacity in downstream purification processes. Chromatography provides a highly selective and scalable alternative to purification techniques such as precipitation and ultracentrifugation. Compared with legacy products, modern chromatography resins offer improved pressure-flow properties that increase productivity. With such features, more product can be produced within a shorter time period, making modern resins more suited for manufacturing applications than legacy products.
In vaccine production, a short time to market is not only beneficial for the manufacturer, but for the patient too.
Flavivirus vaccines
Flaviviruses are complex biomolecular structures, consisting of more than one molecule of protein, nucleic acids, lipids, and carbohydrates. These features of flavivirus poses challenges for vaccine process development and production. Regardless, the optimized production process must yield high recovery and purity, while maintaining integrity of the virus particle. At the same time, the delicate virus particle can be sensitive to commonly used process conditions.
VACCINE TYPES
Flavivirus vaccines can be based on live, attenuated or inactivated virus, recombinant virus subunits, virus-like particles (VLP), or plasmid DNA and viral vectors. Live, attenuated viruses can be produced by modifying the virus in a way that it can infect a foreign host. Modified virus grown in the new host cells results in a population that is different from the initial population. The new population will still grow well in the original host, but will be significantly less virulent. In contrast to inactivated vaccines, an attenuated vaccine contains live, although harmless, virus particles. In inactivated vaccines, cell-based virus particles are inactivated with, for example, betapropiolactone.
Both live, attenuated and inactivated vaccines are whole-virus vaccines, using the whole virus particle to induce an immune response. Basing the vaccine on a virus subunit can be a viable alternative for viruses not easily propagated in cell culture. Vaccines based on virus-like particles (VLP), for example, can be produced by recombinantly expressing antigenic virus proteins, and then allowing them to self-assemble into VLPs (1).
VACCINE PRODUCTION
Egg-based production is a common technique for manufacturing of whole flavivirus vaccines (2). However, egg-based vaccine production is both space- and resource-consuming. Egg-based vaccine production also has its limitations in that it requires a continuous supply of pathogen-free hen eggs. Vaccines produced in hen eggs might also cause allergic reactions in patients with severe egg allergy.
Cell-based vaccine production can be an alternative to meet many of the challenges associated with egg-based production, such as the associated capacity constraints. The challenge with the adherent cell lines commonly used in virus propagation, however, is that they can only proliferate when provided with a suitable surface. Cultivation of adherent cells is traditionally performed in stationary cultures, such as t-flasks, multi-layer flask systems, or roller bottles. To provide sufficient surface area, many roller bottles or t-flasks are required, which is space consuming and makes sampling cumbersome. In addition, roller bottles or flasks offer little or no process control.
A more suitable and reproducible alternative for vaccine production is bioreactor cultivation with microcarriers. Microcarriers offer an increased surface-to-volume ratio compared with rollers/flasks, leading to an enhanced volumetric productivity, while reducing footprint of the manufacturing equipment. The high surface-to-volume ratio also allows waste disposal volumes to be greatly reduced, compared with bottles or flasks. Further, bioreactor cultivation enables automated control of pH and dissolved oxygen, and logging of cell culture data. Sampling of one bioreactor culture is also less labor-intense than sampling of several roller bottles or flask systems. In addition, when using microcarriers part of the culture can easily be sampled for cell quality control or identification.
Stainless steel bioreactors are employed in many vaccine processes. However, such systems require extensive qualification, maintenance, and cleaning, which are all time-consuming activities with a negative impact of the utilization of the production facility. To increase productivity, single-use bioreactor technology is a viable alternative. Reducing the need for cleaning and cleaning validation, changeover between batches and campaigns is quick with single-use equipment.
In addition, construction of a stainless steel-based manufacturing suite is typically more complex and takes a long time. A facility with disposable technology can be constructed much faster than a traditional plant (Fig 1). Single-use equipment also offers increased flexibility compared with stainless steel equipment. Single-use production lines can more easily be modified and production volumes more rapidly adapted to market needs. Allowing for more batches to be produced per year, single-use equipment offers a greater profit opportunity compared with stainless steel equipment (3).
Fig 1. Process train comprising single-use equipment from Cytiva that help accelerate flavivirus vaccine manufacturing. Included systems are suitable for biomanufacturing of regulated products under various quality management systems. The systems are controlled through either Cytiva’s UNICORN or Schneider Electric’s Wonderware® system control software. To enable use of the systems in regulated environments, both UNICORN and Wonderware software are configured to be used in a 21 CFR Part 11 and GAMP 5 compliant manner. All records are stored in a single, unalterable database, including results and extended run documentation. Specially trained and certified engineers perform onsite IQ/OQs and CCPs in accordance with cGMP, as well as provide on-site training for relevant personnel.
The lower initial capital investment associated with single-use equipment is a key driver for use of such a technology, according to the BBC Research report “Single-use technologies for biopharmaceuticals: global markets” from 2013 (4). Another reported benefit with single-use equipment is the reduced cross-contamination risk, as the culture bag is disposed after use.
For downstream virus purification, legacy processes can include separation methods based on precipitation or centrifugation. Such techniques are commonly associated with recovery or purity issues due to poor separation of impurities from the product. These methods can also be difficult to scale.
Separation methods based on chromatography are more suited for manufacturing applications as the technique lends itself for scaling. The excellent pressure-flow properties of modern chromatography resins also allow large quantities to be processed in a short period of time. Additionally, the high selectivity and capacity of these resins enable purification of target entities to high purity and yield.
Addressing shear sensitivity in adherent cultures
Adherent cells are sensitive to shear stress. A rocking bioreactor system provides gentle agitation of the culture to better control shear stress, while providing sufficient aeration of the culture. Single-use rocking bioreactor systems are available for application such as process development, seed culturing, and small-scale productions. Although having a different vessel geometry, studies have shown that rocking systems can give a representative reflection of the process performed in a stirred-tank bioreactor (5). Hence, rocking bioreactor systems can also be used as scale-down bioreactor from a stirred-tank system.
Single-use stirred-tank bioreactor and fermentor systems are based on the same principles as conventional stainless steel bioreactors. Traditional scaling methodology, based on measures such as shear, tip speed, power per unit volume, kLa, and specific process sensitivities, can be used during scale-up. With stirred-tank system platforms, technology transfer is straightforward, minimizing the need for costly and time-consuming process redesign (Fig 2).

Fig 2. Designed for scalability and robustness, the Xcellerex XDR bioreactor system platform provides the performance and flexibility needed from process development to large-scale biopharmaceutical manufacturing. The complete range of XDR bioreactor systems are available with maximum working volumes ranging from 10 to 2000 L, from the smallest XDR-10 to the largest XDR-2000 system.
In bioreactor cultures, microcarriers are used to provide a suitable growth surface for the adherent cells commonly used in virus production (Fig 3). Microcarriers based on a low-density dextran bead enables easy mixing and low shear (6). Bead size and density are optimized to support high cell growth rate and yield. The biologically inert polysaccharide matrix provides a stable, but non-rigid, tissue-like substrate for stirred cultures. Dextran-based microcarriers are translucent, allowing easy microscopic examination of attached cells.
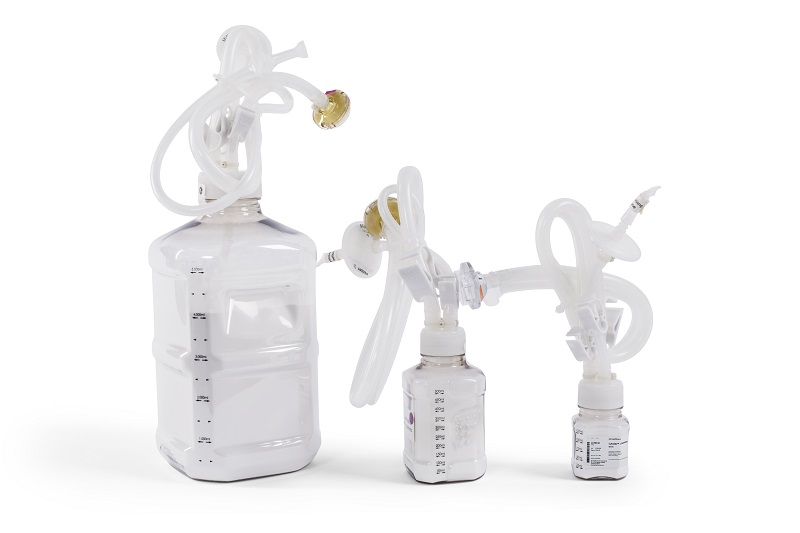
Fig 3.Cytodex Gamma microcarriers are delivered gamma sterilized and ready for use for quick culture startup. In addition, Cytodex Gamma products are supplied dry and shrunken to save storage space and facilitate transportation. To simplify transfer to the cell culture vessel, the container is equipped with flexible connection options.
While many cell lines employed in vaccine production are obligate attachment cells, the EB66® cell line (Valneva), derived from duck embryonic stem cells, grows in serum-free suspension culture at high cell density, allowing for easy and efficient scale-up (Fig 4). EB66 cells form loose aggregate structure that facilitate infection of non-secreted, cell-to-cell transmitted viruses (7).
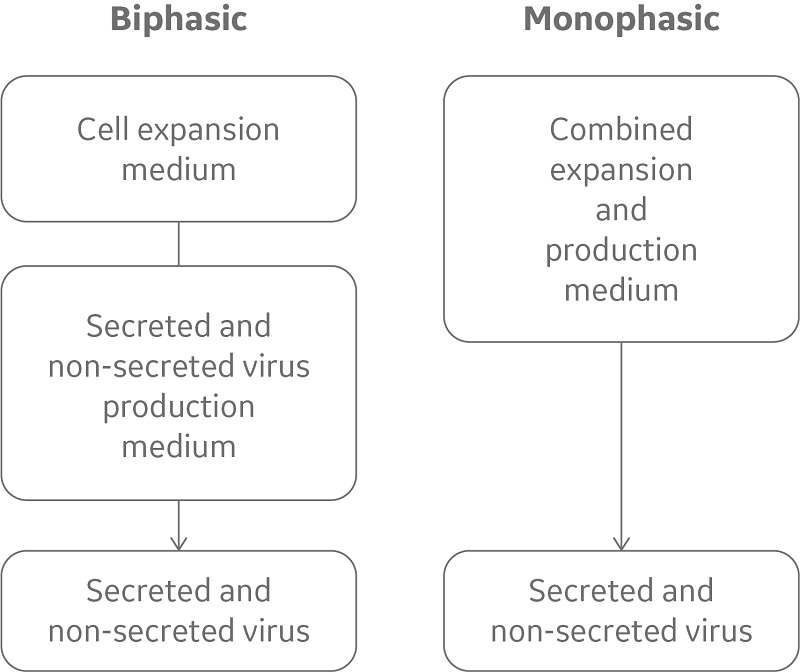
Fig 4. While traditional virus production in EB66 cells is biphasic, requiring two or more media and multiple additives, CDM4Avian medium is designed to support the simpler monophasic approach, requiring fewer additives.
To increase cell density and virus titer, both microcarrier-based adherent and suspension cell cultures can be run in perfusion mode, using a bioreactor equipped with a cell retention filter (8, 9).
Increasing productivity in upstream operations
Selection of the right cell culture medium is important to enhance process yields in the manufacture of viral vaccines. For regulatory readiness, an animal-derived component-free cell culture medium is recommended. Modern culture media are developed to provide optimized conditions for high cell growth and productivity. However, the cell culture medium and feed strategy should be selected with respect to the nutritional requirements of the specific cell clone used. Nutrient concentrations need to be kept within a certain range, as both too high and too low concentrations can be detrimental to the cells.
Design of experiment (DoE) methodologies can be used to perform experiments to identify component groups in the medium that have the greatest effect on cell growth and productivity. This approach produces maximum amount of data with minimum number of experiments, and meets the demands from regulatory authorities for better process understanding, one of the cornerstones of the quality by design (QbD) initiative.
Achieving efficiency in downstream purification
DoE methodology can also be used for identifying parameters affecting purity and yield in downstream processes. Once the chromatography resins are selected, conditions for optimal hcDNA and HCP reduction at maximal product recovery are determined.
Both cation exchange and anion exchange chromatography resins are commonly used in virus vaccine purification processes to reduce impurity levels. There are also examples of affinity chromatography resins with ligands that exhibit affinity for specific viruses such as the adeno-associated virus. For more challenging separations, multimodal resins, with multiple modes of actions (ion exchange, hydrophobic interaction, and hydrogen bonding), can be used. In recent years, a new class of multimodal resins has been developed. In these resins, dual layers have been introduced in the bead design, where size exclusion properties from an inactive outer layer are combined with adsorption chromatography from an ligand-activated core (Fig 5). Small molecules enter the core where they are captured; while viruses and other large entities are excluded and can be collected in the flowthrough.
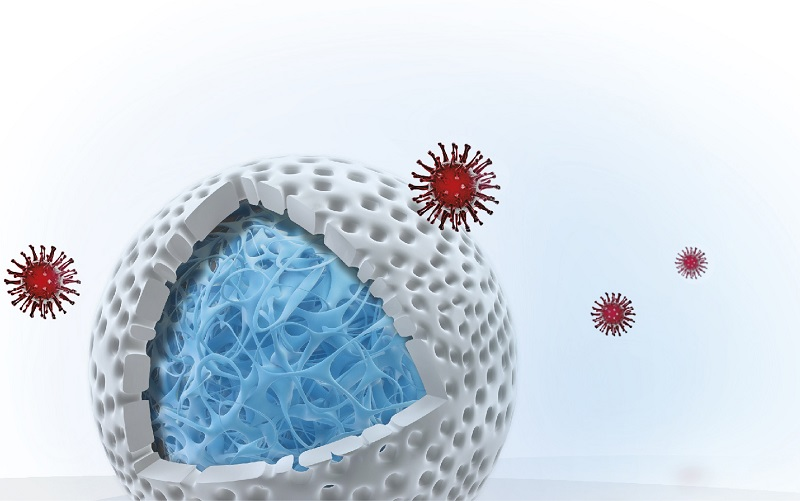
Fig 5. Schematic representation of Capto Core 700 and Capto Core 400 resins, showing their bead with the inactive, porous shell and the ligand-containing core. Proteins and impurities (colored green, yellow, and purple) penetrate the core, while target viruses (red) and larger biomolecules (> Mr 700 000 for Capto Core 700 and > Mr 400 000 for Capto Core 400) are excluded from the resin and pass in the flowthrough.
Modern resins are designed for large-scale chromatographic processes, where high throughput and process economy are essential. Their base matrices have exceptional mechanical stability and optimized pore size to allow efficient capture under high-flow conditions. The improved mechanical stability also increases flexibility in terms of bed heights and the ability to process highly viscous feeds. The chemical stability of these resins ensures a long lifetime, even when harsh cleaning procedures are used. By offering a combination of high volume throughput and high capacity, modern resins provide a powerful solution for fast and efficient processing of large amounts of protein. Where high throughput is of uttermost importance, membrane chromatography is an alternative. Chromatography membranes exhibit a high porosity suitable for virus purification, while providing the opportunity for using high flow rates.
Filtration of delicate targets
Cross flow filtration (CFF), also known as tangential flow filtration (TFF), is a technique extensively used in vaccine production. In contrast to normal flow filtration (NFF), the feed is recirculated over a permeable membrane surface. In CFF, liquid and compounds with molecular weights less than the membrane cut-off can pass through the membrane, whereas larger molecules or particulates are retained and concentrated. For delicate targets, such as the flavivirus, hollow fiber filters are commonly used for the CFF step. Due to the open channel structure, a hollow fiber filter usually causes less damage to the target product compared with a filter cassette (Fig 6). For virus particles expressed in low titers and thus need to be concentrated as much as 200- to 500-times before further processed, single-use tubing assemblies can be used in the design of circuits with low working volumes to enable high concentration factors (11).

Fig 6. Cytiva’s 750 C hollow fiber filter, with a Mr 750 000 nominal molecular weight cutoff (NMWC), is specially designed for use in virus purification workflows, and effectively removes ovalbumin and other proteins in allantoic fluid from egg-based virus production as well as host cell-derived impurities from production in cells. When compared with a 500 C hollow fiber filter, with the same 0.5 mm lumen diameter but with a Mr 500 000 NMWC, in a concentration and diafiltration process, the more open structure of the 750 C filter resulted in a 1.5 to 2 order of magnitude higher host cell DNA (hcDNA) removal at similar host cell protein (HCP) removal and virus yield (10).
Gaining insights with versatile analysis technology
The complex nature of viruses also presents challenges for process analytics. Ideally, analytical methods for vaccine characterization are developed in parallel with process development to aid in gaining regulatory approval and for further manufacturing.
Vaccine design depends upon structural and functional interactions with the host immune system. Label-free molecular interaction analysis based on surface plasmon resonance (SPR) is extensively used in vaccine development and production in areas such as design and characterization, immune response studies, vaccine quantitation, and in analyses during production and quality control (Fig 7).
Fig 7. Vaccine development and manufacturing is supported by Biacore SPR systems, from basic research to production and quality control. Detailed information is obtained from analyses such as binding kinetics, specificity, immune responses, epitope mapping, and concentration.
As has been shown for Zika virus, for example, interaction data can be used to gain insights into the binding of neutralizing antibodies to viral epitopes (12). Using SPR, detailed information can also be obtained from analyses of binding kinetics, specificity, immune responses, epitope mapping, and concentration (13).
Conclusion
Technological challenges can dominate vaccine production. This article gives an overview of modern products and services that can help solve many challenges in flavivirus vaccine production. Bioreactor systems based on single-use technologies support significant time-saving, while increasing process- and operator-safety in cell-based vaccine production. Microcarriers provide the cell surface required for high volumetric productivity of adherent cells in bioreactor cultures. With modern chromatography resins, more product can be purified within a given time frame. Label-free molecular interaction analysis based on SPR technology, can be used for reliable quantification and characterization of the end product. Regulatory-friendly system control software allows equipment to be used in a cGMP-compliant manner.
Modern vaccine production platforms support reduced process time and cost, to help accelerate your flavivirus vaccine production.
Read more about our vaccine platforms
References
- Application note: The use of Capto Core 700 and Capto Q ImpRes in the purification of human papilloma virus like particles. Cytiva, 29098301, Edition AB (2014).
- Application note: Ovalbumin removal in egg-based influenza vaccine production using Capto Core 700. Cytiva, 29103762, Edition AA (2014).
- White paper: Process economy and production capacity using single-use versus stainless steel fermentation equipment. Cytiva, 29143348, Edition AB (2015).
- Report: Single-use technologies for biopharmaceuticals: global markets (ISBN: 0-89336-291-3). BCC Research, Wellesley, MA 02481 (2013).
- Application note: Efficient, high-titer monoclonal antibody production in a fed-batch process using single-use stirred-tank and rocking bioreactor systems. Cytiva, 29119376, Edition AA (2014).
- Application note: Validation of the production of influenza virus in ReadyToProcess WAVE 25 bioreactor system, comparing Cytodex and Cytodex Gamma microcarriers. Cytiva, 29209415, Edition AA (2016).
- Application note: Virus production in suspension-adapted avian cells using a chemically defined medium. Cytiva, KA968041017AN (2017).
- Nikolay, A., Hermann, K., Genzel, Y., Reichl, U. Evaluation of producer cell lines in yellow fever virus production in up to 1 L bioreactor scale. Poster at Vaccine Technology VI, June 12–17 (2016).
- Nikolay, A., Castilho, L., Tanuri, A., Reichl, U, Genzel, Y. Propagation of Brazilian Zika virus strains in static, microcarrier-based and suspension cultures using BHK and Vero cells. Poster at Vaccine Technology VI, June 12–17 (2016)
- Application note: Concentration and diafiltration of cell-derived, live influenza virus using 750 C hollow fiber filter cartridge. Cytiva, 29092826, Edition AA (2014).
- Application note: Efficient concentration of a low-titer bovine IgG with high recovery in low volume. Cytiva, 29228378, Edition AA (2016).
- Dai, L., Song, J., Lu, X., Deng, Y-Q, Musyoki, A.M., Cheng, H., Zhang, Y., Yuan, Y., Song, H., Haywood, J., Xiao, H., Yan, J., Shi, Y., Qin, C-F., Qi, J., Gao, G.F. Structures of the Zika virus envelope protein and its complex with a flavivirus broadly protective antibody. Cell Host & Microbe19, 696–704 (2016).
- White paper: Biacore systems in vaccine development and production. Cytiva, 28987028, Edition AB (2016).