Buffer preparation is one of the most resource-intensive activities in biomanufacturing due to the large number and overall volume of buffers and process liquids used in a typical bioprocess workflow. As a result, buffer preparation can be prone to create bottlenecks in the manufacturing process. Here we present different approaches to resolve buffer management challenges in large-scale biomanufacturing.
This article discusses how outsourcing buffers and technologies such as inline conditioning (IC) and inline dilution (ILD) can help prevent resource constraints, save time, and reduce manufacturing footprint and overall cost in buffer preparation.
As an example, we demonstrate how IC can be used to reduce the total volume of the stock solutions up to 79% and the total footprint of the buffer management system and tanks by 40% over traditional buffer preparation.
Introduction: the need for adding capacity to buffer management
Manufacturing of biotherapeutics requires large volumes of process liquids. A 2000 L production batch of a monoclonal antibody at a titer of about 5 g/L is estimated to use 16 000 to 22 000 L of water for injection (WFI).
Such volumes, coupled with the large number of different types of buffers and process liquids that are used in bioproduction, can exert pressure on in-house buffer preparation operations and lead to capacity constraints. As a result, biomanufacturers are looking for ways to add more capacity to their buffer preparation without major capital investments.
Buffer preparation drives cost
Buffer preparation is a highly manual and resource intensive activity. Also, the large holding tanks require significant floor space that could be used for other core operations. By efficient use of existing stainless steel facilities, cost of in-house buffer preparation and other process liquids is relatively low compared with preparation using single-use equipment or outsourcing.
Challenges, however, arise when buffer capacity has reached its maximum and needs to be expanded or when the capacity is not fully utilized. In addition, biomanufacturers must consider how current capacity aligns with their product pipeline for drug candidates that may or may not achieve commercial approval.
Hence, it can be beneficial to postpone investments in in-house facilities and instead outsource buffer production until the outcome of clinical trials has been evaluated.
Trends in outsourcing buffer preparation
Outsourcing buffer preparation is a simple and fast way to gain extra or new capacity. It is, however, still relatively rare in the industry, but a recent report shows that it is becoming more prevalent for biomanufacturers and it has become a key area of interest. (1) There will be a slowly growing market for outsourcing, particularly among new single-use facilities and continuous chromatography unit processes.
These generally ”downsized” facilities are prime candidates for adopting the outsourcing strategy. Also, it is likely that the increasing number of new retrofitted facilities will adopt buffer fluids outsourcing, perceiving this as being advantageous for speed-to-market and flexibility.
So, outsourcing can be a good solution especially for biomanufacturers operating at smaller scales. In addition to enabling a smaller facility footprint, the ability of a supplier to handle quality assurance (QA) and quality control (QC) can further lessen the burden on the manufacturer. (2)
Added buffer capacity with outsourced buffers
Outsourcing buffer production requires some initial efforts for activities such as qualifying the supplier and setting specifications for raw materials and containers. However, the advantages gained by outsourcing include leveraging the supplier’s manufacturing capabilities, supply chain, logistics expertise, and QA/QC teams (Fig 1).
Fig 1. Advantages of outsourcing buffer preparation.
Once the initial work on vendor validation has been done, the biomanufacturer continues to benefit from a robust supply chain through reduction in buffer preparation and quality control testing time as well as QA documentation, all of which result in a reduced audit burden.
Technologies for automated buffer preparation
Another way to overcome buffer challenges is the use of technologies such as inline conditioning and in-line dilution. In these technologies, buffers are prepared in line from buffer concentrates or from highly concentrated, low- volume, single-component stock solutions.
This way, buffer preparation can be directly integrated with the chromatography or filtration step, eliminating the need for intermediate storage in buffer bags or holding tanks.
In addition to reducing facility footprint, automating the buffer preparation process also ensures the quality of the final formulation. Integrated inline buffer preparation supports the industry’s need to streamline processes, increase productivity, and utilize facilities more efficiently.
Intensified processes with inline buffer preparation
By implementation of techniques such as ILD and IC, buffer production remains an in-house activity, but labor and space required for buffer preparing and handling are reduced by automating the preparation process and using concentrates to reduce buffer volumes.
With ILD, volumes are reduced by using buffer concentrates that are diluted in line with WFI-grade water (Fig 2). Using IC, buffers are prepared in line from concentrated, single-component stock solutions of acid, base, salt, and WFI (Fig 3).
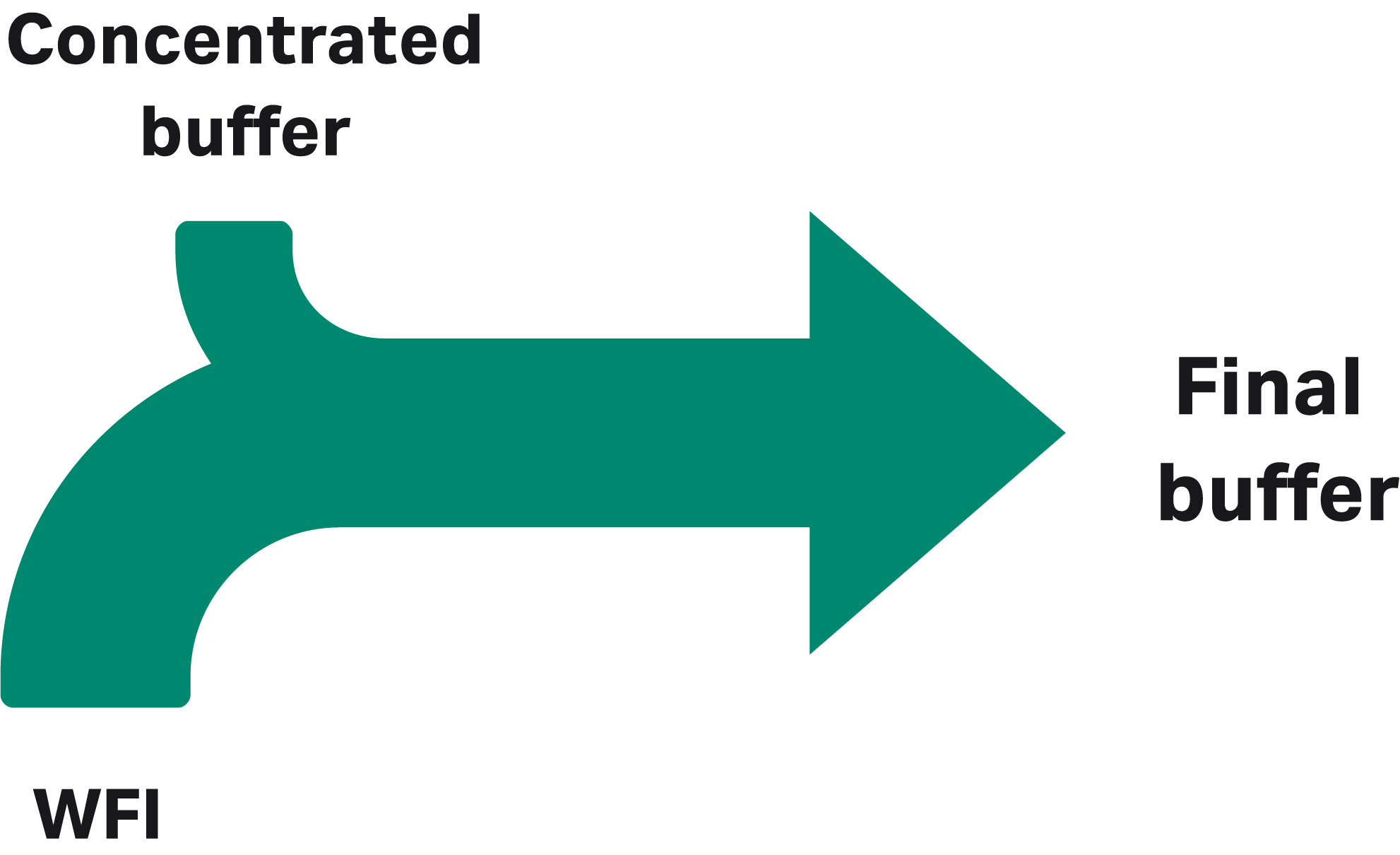
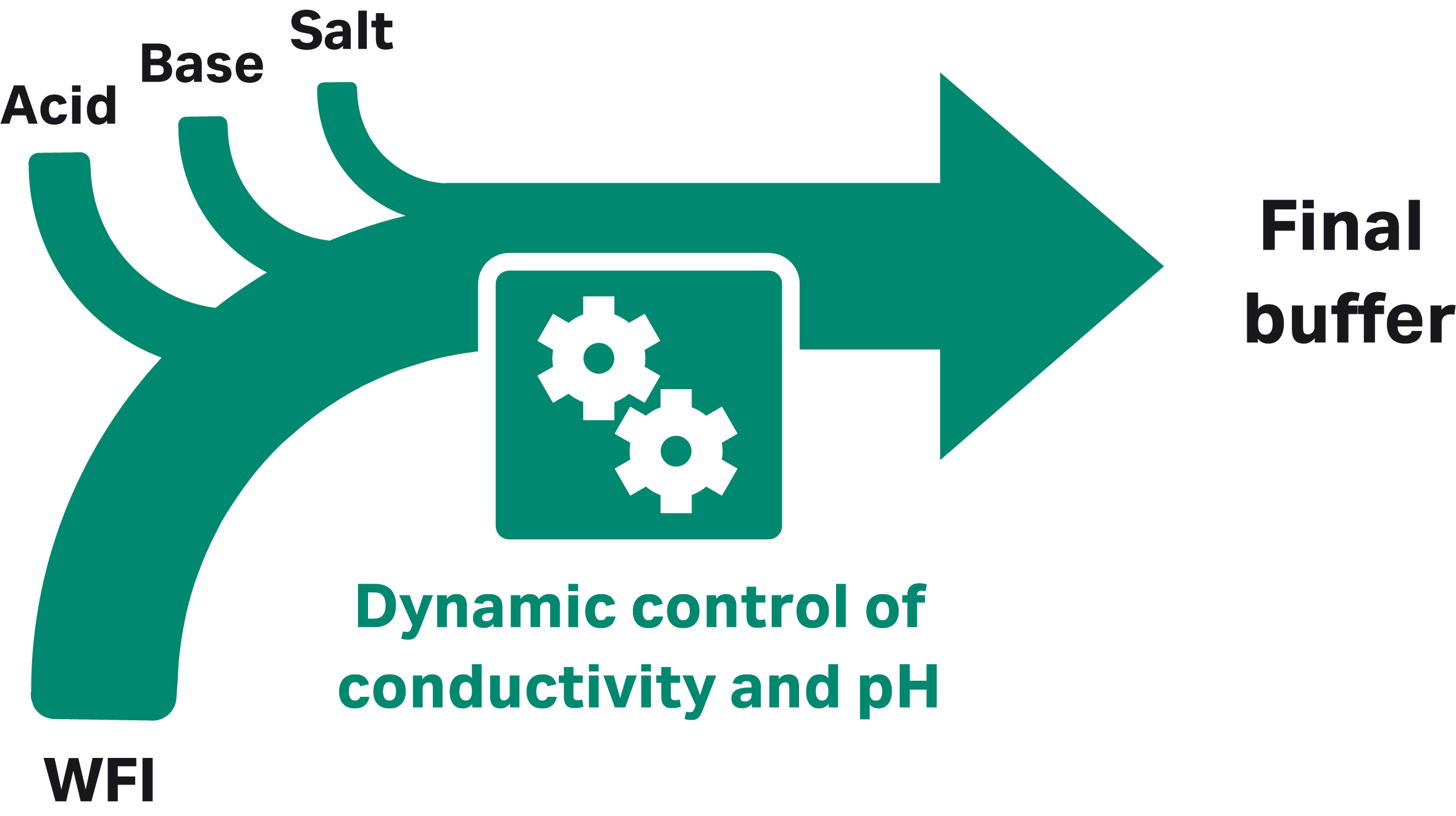
Fig 2. Inline dilution uses concentrated, well-defined buffers as input.
Fig 3. Inline conditioning uses stock solutions of buffer components as input.
In ILD, one concentrate is required for each individual buffer used in a specific process, whereas for IC, a few stock solutions can be used to prepare all buffers needed for the process. With both ILD and IC, significant reductions in floor space and tank volumes can be achieved (Table 1).
However, the largest space reduction comes with IC, as single-component stock solutions can be much more concentrated than a multi-component buffer concentrate, of which the least soluble ion will limit its maximum concentration.
Table 1. Comparison of buffer management options
| Technique | Advantages | Drawbacks |
| Inline dilution | Significant reductions in floor space and tank volumes. Increased automation. | pH shifts due to dilution have to be handled. Sensitive to least soluble component. Affected by common ion effect (CIE). |
| Inline conditioning | Significant reductions in floor space and tank volumes. Reduced operator handling and increased automation. Can condition many buffers from same stock solutions (steps, gradients, etc.). Dynamic control: use combinations of various types of feedback control. The least soluble component affects only itself and not affected by CIE. | System investment cost can be perceived as high. More extensive training is required. |
| Outsourced premade buffers or stock solutions | Ability to leverage the supplier’s manufacturing capabilities, supply chain, logistics expertise, and QA/QC teams. Immediate capacity relief, readily available products. Customer-driven formulations to allow custom solutions to fit the process or facility footprint. Allows for scalability. Variability of buffer preparation is reduced. | Requires some initial efforts such as qualifying the supplier and setting specifications. Additional logistics considerations required to order, receive, and use buffers as needed. |
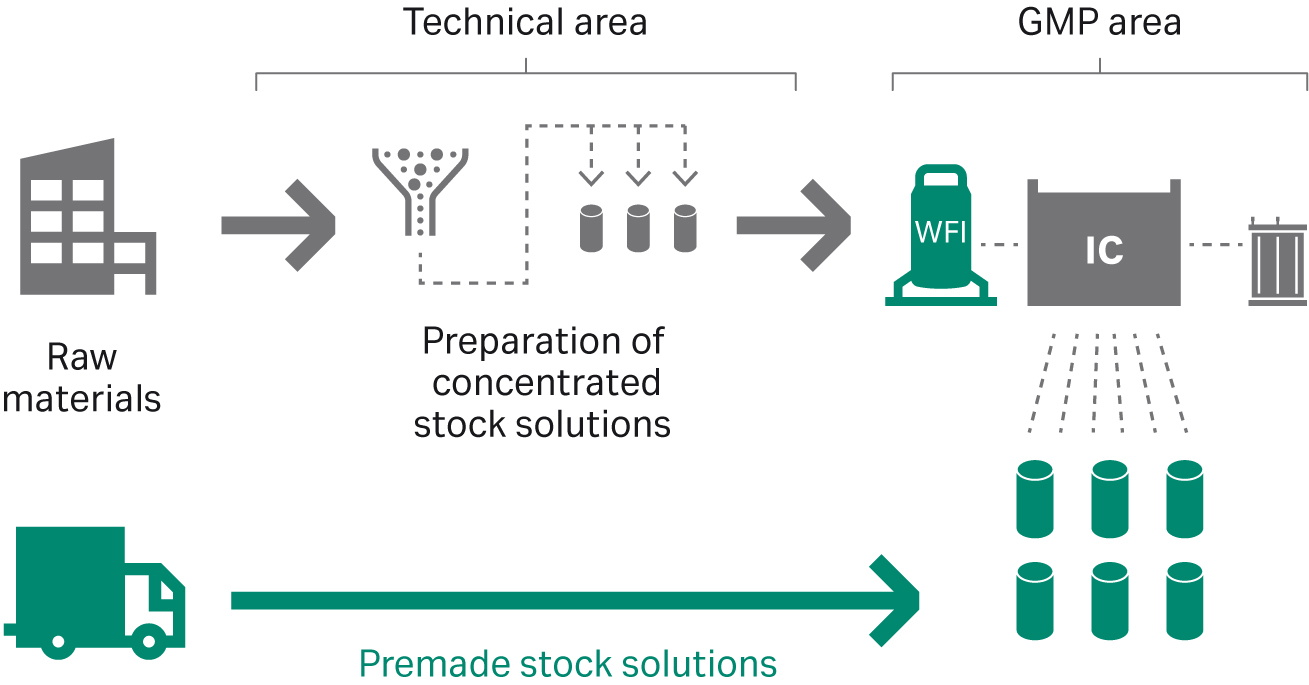
The use of single-component stock solutions as inputs provides additional benefits in that they are more straightforward to prepare than buffers or buffer concentrates, as they require dissolving of one single component only and no need for titration and pH adjustment. As an alternative to manually preparing buffer concentrates or single-component stock solutions in house, production of the input solutions can also be outsourced (Fig 4).
Fig 4. Outsourcing preparation of stock solutions enables great time savings and enhance resource utilization.
For standard 1× buffers, there is a practical batch size limitation, as the largest available single-use bags are around 3000 L. This volume is generally not sufficient for biomanufacturing applications. From this perspective, technologies such as ILD and IC also facilitate the transition to single-use solutions due to the reduction in input volumes.
Implementing technologies such as ILD and IC help streamline the entire buffer preparation process and reduce manual handling by automating several steps, not only reducing the risk of human errors, but also making it possible to reassign personnel to other tasks that provide more value.
Integrating buffer preparation with unit operations
IC systems and more advanced ILD systems have the functionality required to operate as a chromatography unit. This functionality allows direct connection to a chromatography column to make it possible to deliver the buffers directly to the process without the need for storage in bags or tanks.
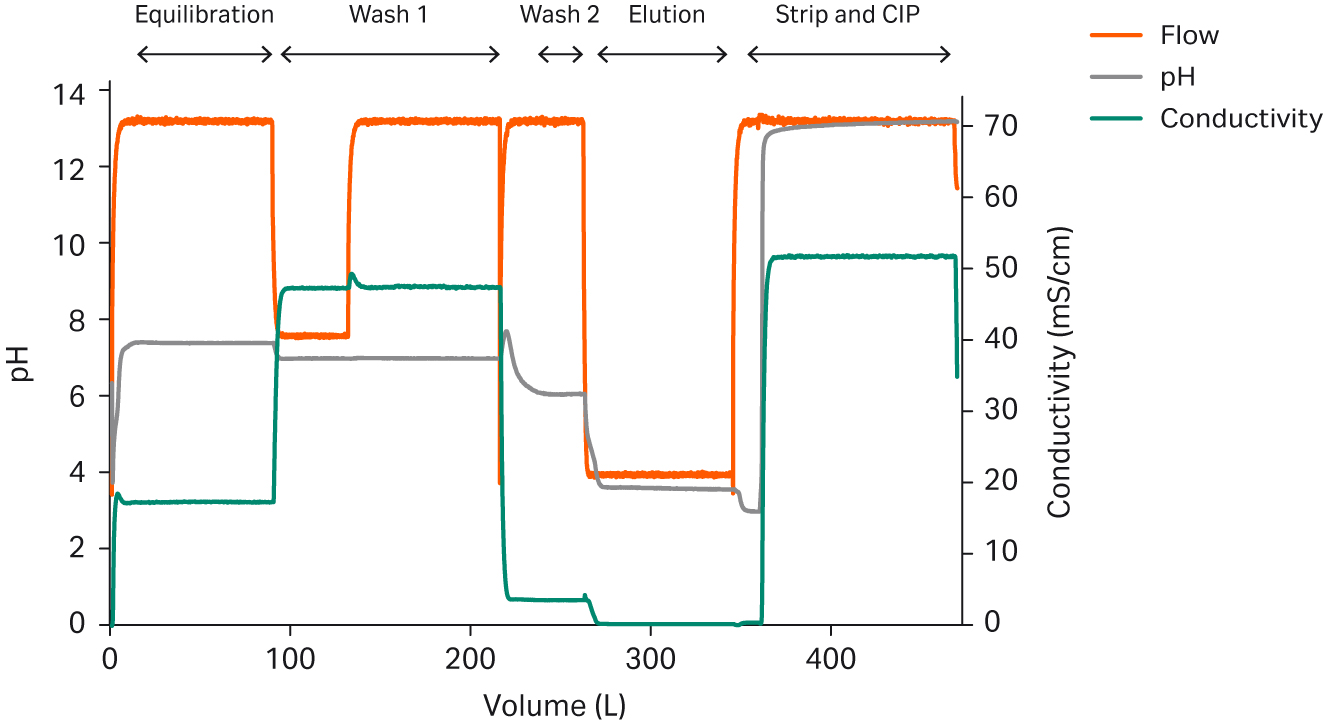
Furthermore, with IC, the system will prepare the exact amounts of the relevant buffers in line from the stock solutions. Waste will only be generated during the switch between buffers until the set pH is reached and stable (Fig 5).
Fig 5. Inline conditioning used in preparation of buffers as well as strip and clean-in-place (CIP) solutions required for a mAb capture step. The arrows indicate preparation of formulations within specifications.
Moving IC into the technical area
As IC uses single-component stock solutions as inputs, this technology can also be transferred into the technical area and serve as a stand-alone buffer production unit. The system has dedicated pump lines for acids, bases, and salts, with several inlet ports to enable formulation of different buffer families in one production run, providing a high degree of flexibility.
Ensuring quality rigor in buffer preparation
IC control functions
Because buffers are used to maintain purification conditions as well as stabilize the bioproduct and preserve its functional characteristics, correct buffer formulation is crucial for success in bioproduction. Usually, a buffer is defined by a set of parameters that typically are critical process attributes (CPAs) of which pH and conductivity are the most obvious.
However, there might be additional parameters that are important for the process, such as temperature, buffer concentration, and concentration of additives. If the buffer is a mixture of components, the concentration of each component can be critical. If a post-adjustment approach is used, having full control of these additional parameters can be challenging.
With IC, it is possible to select the feedback mode that best controls critical process parameters (Table 2). Feedback control will also ensure that the mass balance is kept. There are three modes of feedback control that can be used with the dynamic control functionality of the system: recipe and flow; pH and flow; and pH and conductivity.
Table 2. Control modes used with IC
| Control mode | Benefits | Drawbacks |
| Recipe and flow | Robust if temperature is constant. Can use recipe generated from pH and flow feedback. |
Can lead to variations in pH and conductivity if temperature varies. Need accurate stock solutions. |
| pH and flow | Correct pH even if temperature varies. Generates recipe to be used in flow feedback. |
Sensitive to bias in pH meter. |
| pH and conductivity | Correct pH even if temperature varies. Correct conductivity even if temperature varies. Does not need accurate stock solutions. |
Sensitive to bias in pH meter. Sensitive to bias in conductivity meter. |
Case studies on automated buffer preparation
Accuracy in buffer formulations using inline conditioning
This study describes a lean approach to buffer preparation by implementing IC. Buffers of different formulations for a mAb chromatography capture step were prepared in an automated, consecutive manner using an inline conditioning system.
To further reduce the time and space required for buffer preparation, HyClone ready-made, highly concentrated, low-volume, single-component stock solutions were used. The dynamic control functionality was used for feedback regulation of the final buffers to ensure accurate formulations.
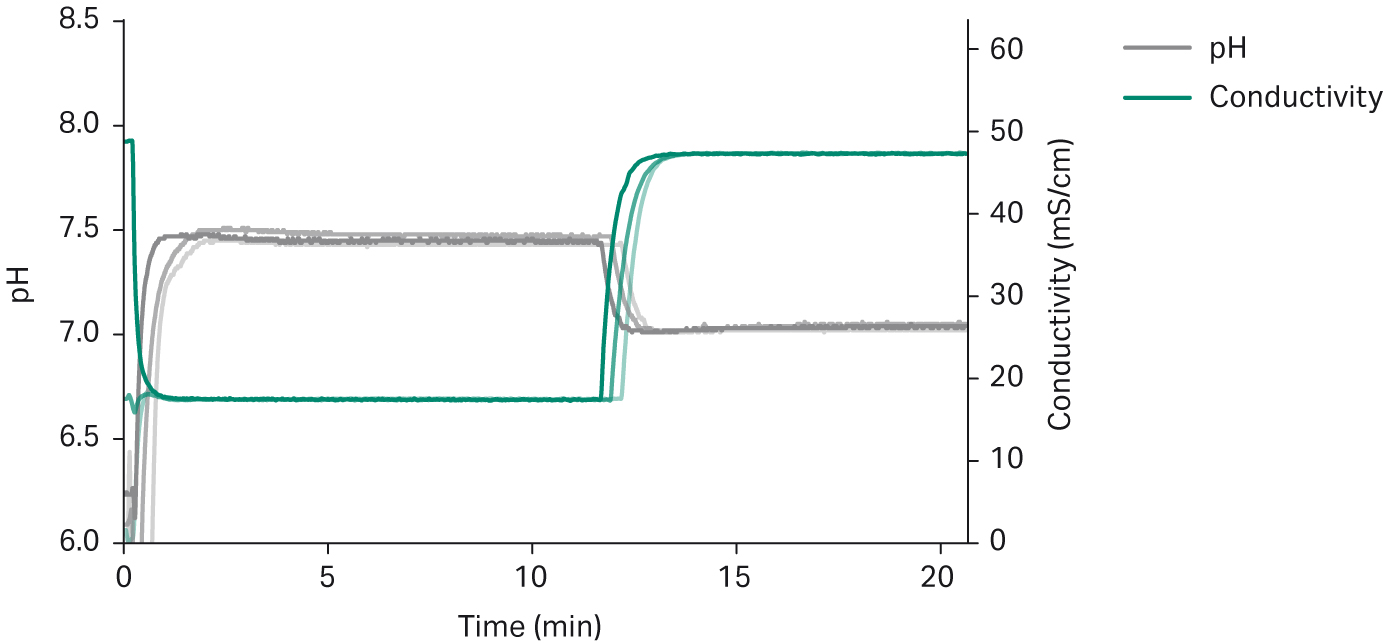
Tough pH and conductivity accuracy requirements in bioprocessing put high demands on the robustness of the system performance between batches and runs. In Figure 6, an overlay of three preparations of two buffers with an intermediate switch between buffers demonstrates consistency between the preparations.
Fig 6. Overlay of triplicate preparations of 20 mM sodium phosphate, 150 mM NaCl, pH 7.4 buffer, followed by preparation of 20 mM sodium phosphate 500 mM NaCl, pH 7 buffer, showing reproducibility of buffer formulation. The time to switch from one formulation to another is similar between preparations and takes about two minutes.
Read the full story on automated inline buffer preparation from ready-made stock solutions in a mAb process step.
Process economy using IC versus manual buffer preparation
We conducted a process economy simulation comparing traditional manual buffer preparation with automated buffer preparation using IC. The objective was to compare volumes needed to formulate buffers and the footprint required, as well as cumulative running costs for different approaches.
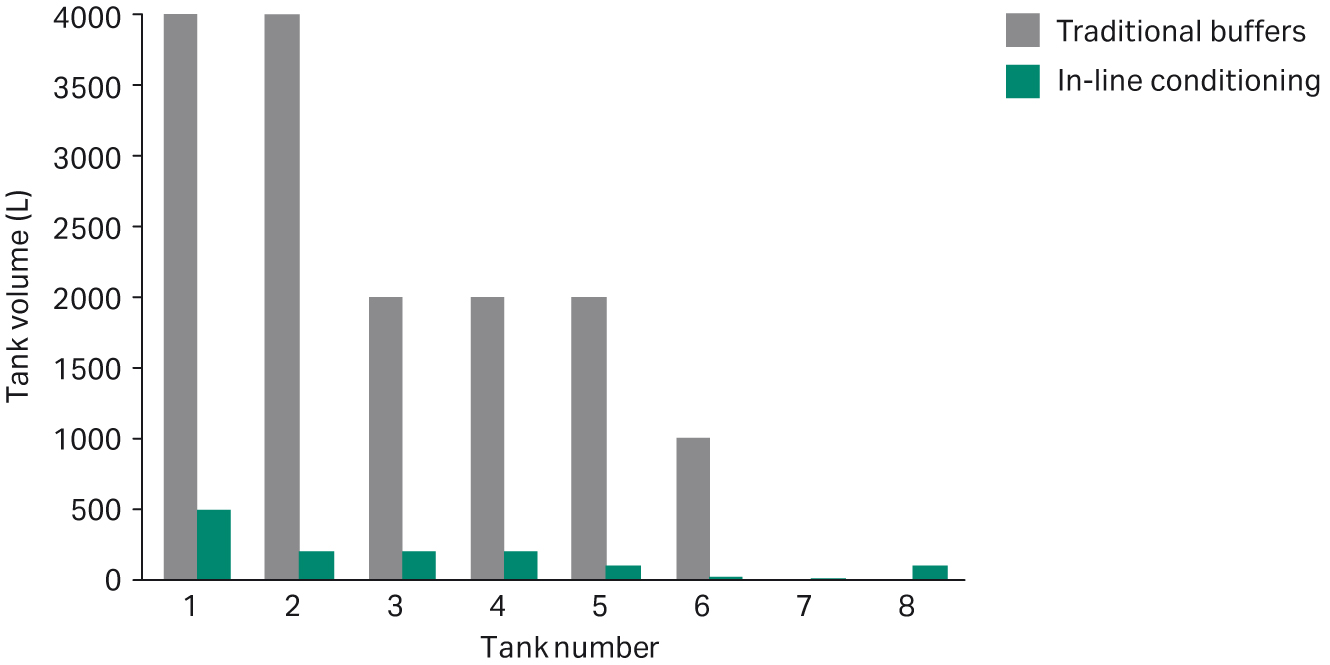
Compared with the traditional way of preparing buffers, the results show that the volume of the buffer holding tanks can be reduced by up to 90% by using IC (Fig 7). In addition, the total footprint of tanks and system can be reduced by 40%.
Fig 7. Comparison of tank volumes between traditionally prepared buffers and buffers prepared using IC.
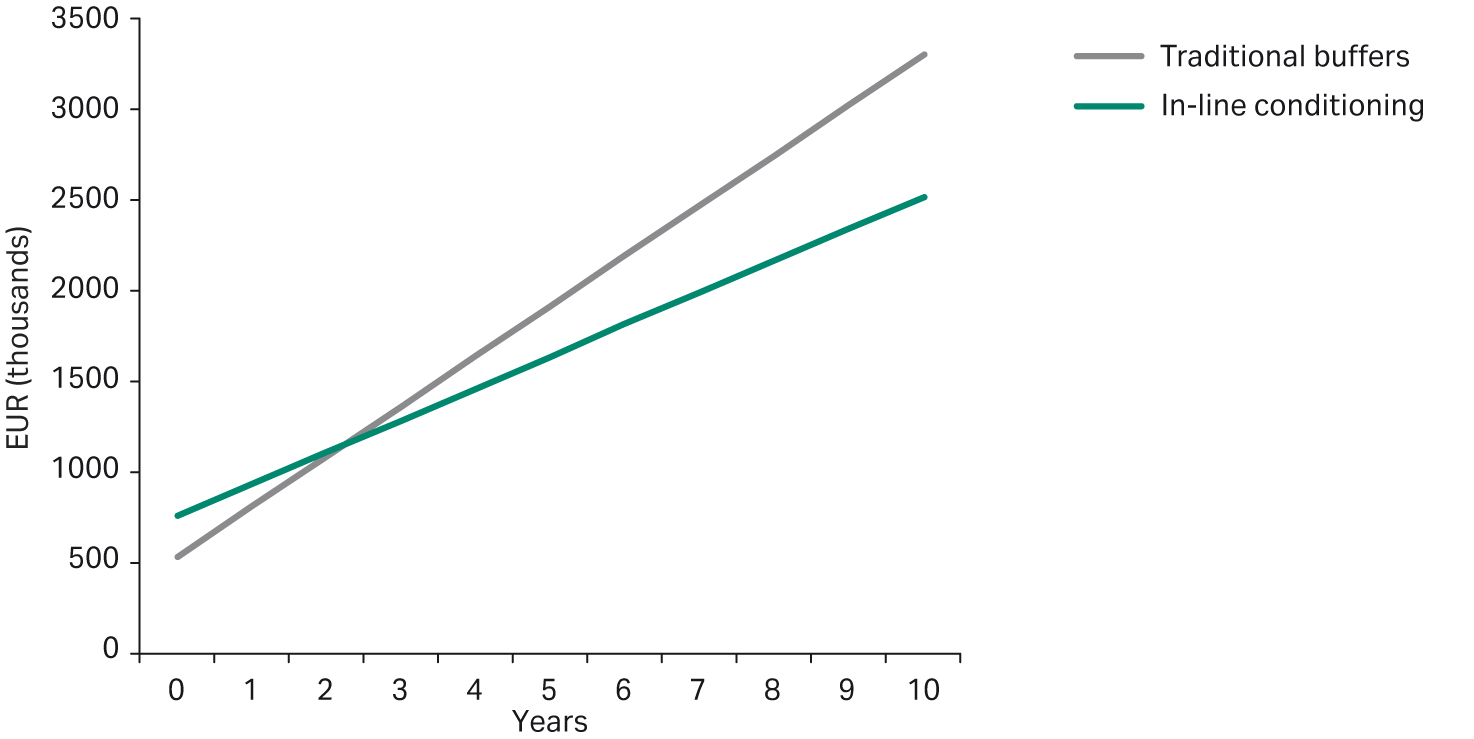
Although the initial investment, including tanks and system, is larger for IC, the savings in operating costs are significant. As shown in Figure 8, the higher investment cost for IC can be recouped after only a few years of operation.
Fig 8. Comparing accumulative running cost between traditionally prepared buffers and buffers prepared by IC.
Read the full case study on how to overcome buffer challenges with inline conditioning.
Conclusion
When evaluating ways to save costs in buffer management, it is important to both study the amount of time and cost spent on buffer preparation and take a wider look at the productivity of the facility’s entire footprint.
Space needed for in-house buffer preparation, inventory, and storage is disproportionally large, but does not add directly to the overall productivity. In addition, any bottleneck experienced in buffer preparation can reduce production capacity utilization.
By reducing footprint and relieving resources dedicated to buffers preparation, biomanufacturers can improve overall productivity. At a first glance, the purchase of ready-made buffers and other process liquids can appear more costly than in-house prepared equivalents. However, a mix of outsourced and in-house buffer preparation can often provide the best solution.
In larger scale, technologies such as IC and ILD are efficient options for buffer preparation, and the use of ready-made, low-volume stock solutions can make the process even more efficient. By allowing for integrated, just-in-time buffer preparation, IC and ILD eliminate hold time as well as the need for intermediate holding tanks between process steps.
Interested in more in-depth details and a view on the importance of security of supply for buffer management? Download the white paper Buffer management solutions for large-scale biomanufacturing.
- Report and survey of biopharmaceutical manufacturing capacity and production, BioPlan (2019)
- Bioprocessing buffers and related fluids: market opportunities and analysis, Bioplan, commissioned by Cytiva (2017)