Antibody fragments such as single domain antibodies (dAbs), antigen-binding fragments (Fab), and single chain variable fragments (scFvs) have unique properties that can make them better suited to certain therapeutic applications than full-sized monoclonal antibodies (mAbs). Fragments are smaller than mAbs, which can allow them to more effectively reach their targets, and they’re also often easier and less expensive to produce. But purification of antibody fragments isn’t as straightforward as for mAbs — fragments show wider variation in binding affinities and can be trickier to separate from impurities. Here we’ll look at some purification approaches that can help you harness the potential of antibody fragments.
What types of antibody fragments are used in bioprocessing?
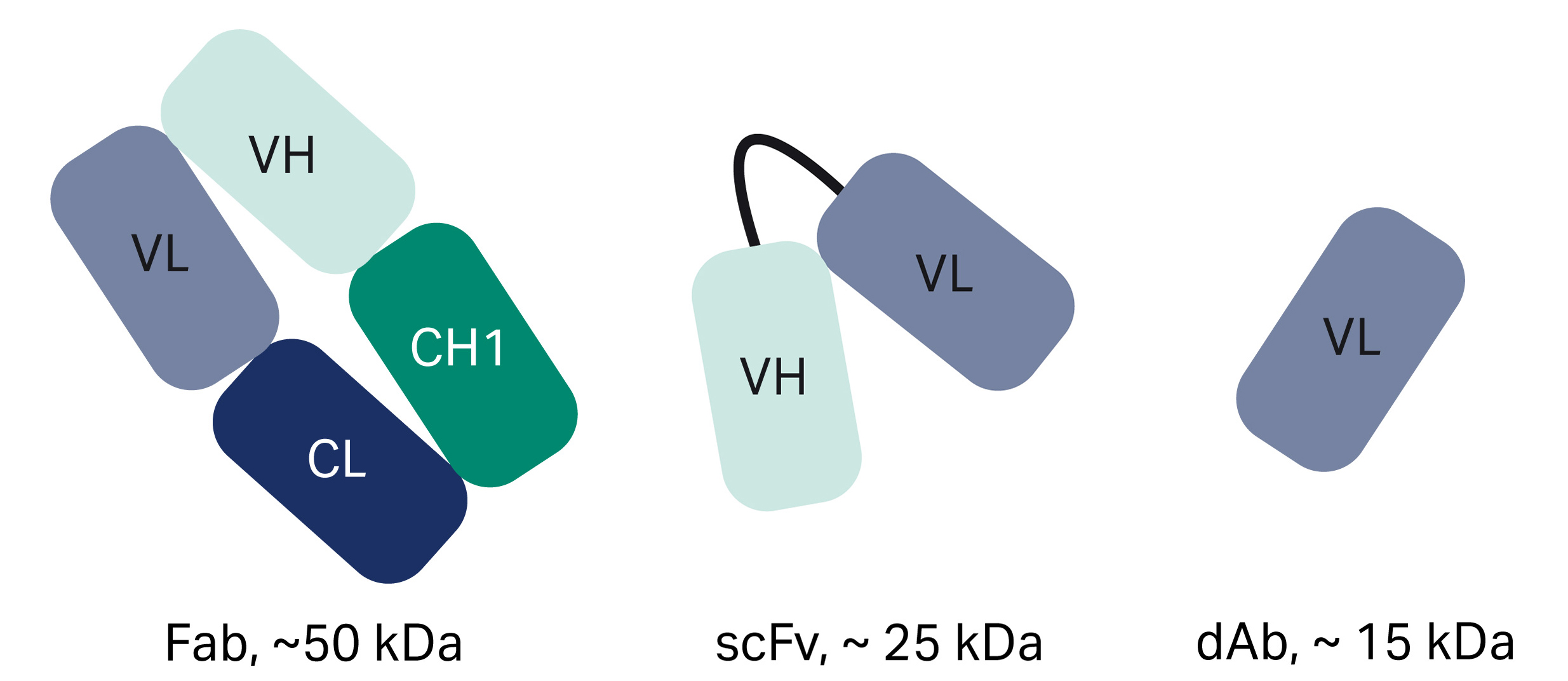
Fabs are antibody structures that bind antigens but lack an Fc region. Considered the first generation of antibody fragments, Fabs were first generated by enzymatic cleavage of an intact antibody to yield an Fc fragment and two Fab fragments, each composed of one variable heavy chain (VH) and one variable light chain (VL). Today, Fab fragments are produced using genetic engineering and can be generated by simpler expression systems, such as E. coli.
scFvs are recombinant monovalent structures with affinity for a single antigen. With an approximate size of Mr 25 000, an scFv is a fusion of the VH and VL chains. An scFv comprises the complete antigen-binding site of its parent antibody molecule.
Single domain antibodies (dAbs) are some of the smallest functional antibody fragments that retain full antigen-binding specificity. They consist of the VH or VL domains and are around one-tenth of the molecular weight of a full-sized antibody. dAbs are stable under harsh conditions of temperature, pressure, and denaturing chemicals.
Production and purification of antibody fragments
Antibody production workflows involve expression of the protein in mammalian or bacterial cells, followed by downstream clarification of the culture to remove cellular material, then antibody capture and polishing. Here we’ll look at options for each step.
Antibody fragment expression
Because antibody fragments are small and not glycosylated, you can use bacterial or yeast cell expression systems to produce them, making them simpler and less expensive to make than mAbs. Tangential flow filtration (TFF) with hollow fiber filters is a good option for clarifying viscous and high-solid feeds after microbial fermentation.
Antibody fragment purification
Typically, mAbs can be purified using a platform approach, where standard unit operations and operating conditions are constant. The Fc region common to mAbs means that a near-generic approach is possible. Fab fragments, scFvs, and single domain antibodies require a broader strategy, but newer affinity resins open efficient capture alternatives. We offer a variety of chromatography resins suitable for antibody fragment purification, including Fabs, scFvs, and single domain antibody lambda and kappa light chains. Your approach for the capture chromatography step will depend on the structure and properties of your fragment.
Capturing fragments containing kappa light chains
Because of its binding specificities, protein L has broad potential as an affinity ligand for antibody fragments. Native protein L interacts with immunoglobulin (Ig) kappa light chains and will bind to several antibody classes, including IgG, IgM, IgA, IgE, and IgD. It has no immunoglobulin class restrictions. Approximately 60% of mammalian IgG light chains are kappa chains with protein L binding activity (1).
Where previous generations of protein L ligands lacked the binding capacity and alkaline stability needed for industrial scale purifications, newer ligands, like the one in MabSelect™ VL resin, overcome those challenges. MabSelect™ VL resin includes a rigid base matrix, allowing for high flow rates and high productivity as well as low ligand leakage and suitability for large-scale manufacturing. Its broad affinity for a range of antibody fragments of different sizes that contain the variable region of the kappa light chains is useful for a wide range of purification applications for Fabs, scFv, and dAbs as well as for bispecific antibodies.
KappaSelect affinity resin binds to the constant region of the kappa light chain and can be used to capture relevant Fabs in conditions where MabSelect™ VL resin is not suitable.
Capturing fragments containing lambda light chains
For capture of antibody Fabs containing the lambda light chain, choose LambdaFabSelect.
Capturing fragments containing heavy chains
If you want to capture heavy chain fragments that contain the VH3 domain subtype, you might want to consider MabSelect PrismA™ or Fibro PrismA. The PrismA protein A ligand binds to the VH3 domain subtype of human IgG Fabs in addition to binding in the Fc region, making it suitable for some antibody fragment purifications.
Intermediate purification and final polishing
For intermediate fragment purification and final polishing, separation based on different selectivity from the primary technique (orthogonal) can involve anion exchange (AIEX) or hydrophobic interaction chromatography (HIC). Your intermediate and polishing steps are based on impurity levels after capture. These steps reduce aggregates, HCP, and endotoxin and can be run in bind/elute or flowthrough mode.
Learn more about antibody fragments
- Protein A and protein L chromatography for protein purification
References
- De Château M et al. On the interaction between protein L and immunoglobulins of various mammalian species. Scand J Immunol. 1993;37: 399-405.