By Sofie Stille, GM BioProcess Resins & Technologies and Elles Steensma, Program leader, Cytiva
Today, biologics represent a >$300 billion industry, which is two times what it was worth only 10 years ago (1). It is estimated that in just four to five years biologics will make up over 50 percent of the 100 top-selling drugs, corresponding to over a third of the total market (2). Though monoclonal antibodies are expected to continue dominating the market, a more diversified pipeline that includes, for example, DNA and RNA molecules and cell and gene therapies is anticipated as the growth continues.
Responding to the rapidly changing requirements of these novel therapies means tackling some of the biggest challenges that have the potential to hinder this evolution. One such area is sustainability and security in the biopharmaceutical supply chain.
The Maturation Of the Supplier/Manufacturer Relationship
Compared to just a decade ago, managing the biopharmaceutical supply chain has become a far more complex topic. Many factors are contributing to the complexity, including the increased molecular diversity and drug volumes, as well as the enhanced regulatory requirements and increased use of single-use consumables, system components, and chemicals. Also, companies are merging and splitting more frequently, which often requires additional auditing of supply networks.
Suppliers and manufacturers have to handle the supply chain complexity better. This begins with transforming relationships that are historically transactional to those that drive suppliers to become value-add partners.
Yet, regardless of whether we are biomanufacturers or suppliers, we must give serious thought on how to handle the supply chain complexity better, not just for the continued financial health of the industry but also in the interest of the good health and lives of patients. This begins with transforming relationships that are historically transactional to those that drive suppliers to become value-add partners working toward shared goals with their customers.
Barriers built over time must be broken down and, in their place, bridges created where suppliers, biopharmaceutical companies, and regulators acknowledge their interconnectedness and work together toward more reliable, efficient supply chain operations. This establishes not only continuity in supply exchange but also proactively prepares the industry for costly, and sometimes dangerous, interruptions.
For example, the 2011 tsunami in Japan disrupted the supply of a single-sourced, critical raw material that Cytiva uses in its chromatography resin called Sephacryl and that our customers use in the manufacturing of registered biopharmaceuticals. Also, the volcanic eruptions in Iceland disrupting supply logistics, the raw material scandal with heparin, and the trade tension between the US and China have shown the industry that supply incidents can be diverse.
The global expansion of the biopharmaceutical business has also changed our supply “chain” into a supply “network,” requiring a deeper level of transparency and control from all stakeholders. Historically, managing supply meant gaining a deep understanding of your primary supplier; however, having control over your supply today means understanding not just your primary suppliers but the relationships they have with their suppliers, often going even two to three levels down.
This more holistic approach provides insight into how different materials and components travel through the supply network as well as controlling and understanding your raw materials, so you can design robust processes that account for potential variabilities. It also means customers need to share information, so suppliers can better understand how their raw material quality attributes interact with customers’ process requirements. This requires in-depth collaboration and experimentation.
The supplier network that is required to manufacture products for the biopharmaceutical industry is increasingly complex with many interdependencies. That is why it is necessary to take a data-driven approach to understand the risks manifest in a supply network.
Today, Cytiva has many teams across the organization working with security of supply to understand which properties are critical to the products that we manufacture and what kind of impact raw materials can have on manufacturing processes. Where needed, validation groups build suitable validation strategies, including for changes with ingoing raw materials. Sourcing qualifies and analyzes our suppliers to understand their capacities and potential risks, and manufacturing ensures that we can safely scale up process volumes without altering the product quality.
Operational Improvements Through Transformative Supply Strategies
Raw material variability is one of the most recent additions to Cytiva's Security of Supply toolbox, which is a comprehensive risk management strategy for the safe delivery of drugs even in the face of unforeseen or disruptive circumstances. Because each product portfolio in bioprocessing has unique supply chain, quality, and manufacturing needs that require different solutions, it is critical to create a holistic approach to providing high-quality products for the lifetime of the drug. Cytiva's Security of Supply program encompasses three main elements: supply chain sustainability, business continuity, and communication.
Supply chain sustainability helps control the supply chain by identifying any raw materials, consumables, and components needed to manufacture a product and then deciding on what actions are necessary to ensure a consistent, reliable supply of those materials. Our supply risk management program is used to reduce the number of high-risk suppliers and to identify resource-effective activities to mitigate supplier risks.
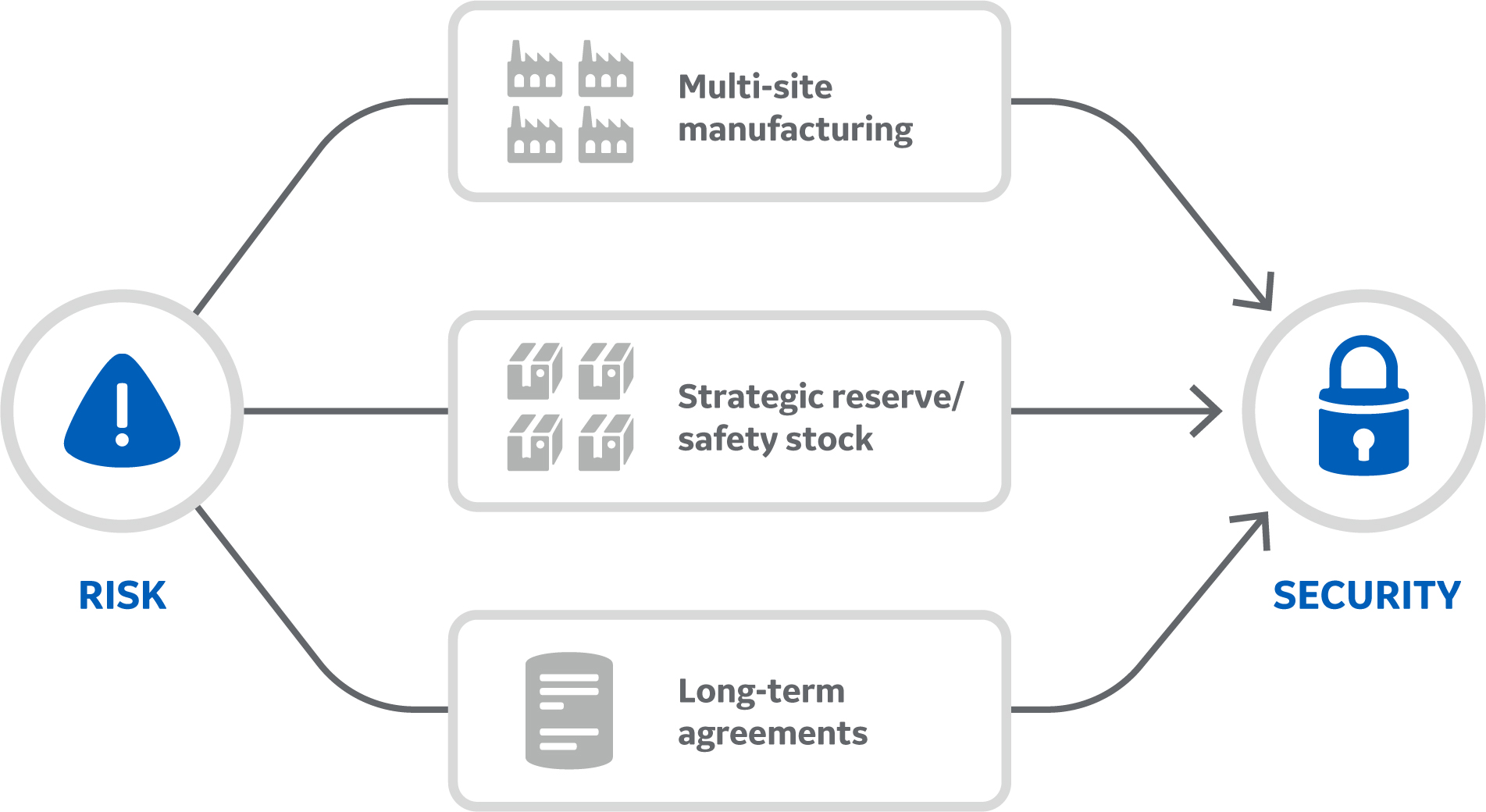
The business continuity management program focuses on our own manufacturing operations by providing a framework for plans to reduce risks and costly downtime after a potential incident in our facilities. This approach ensures a consistent, reliable supply of products to our customers. Concrete examples include multi-site manufacturing, safety stock, and forming long-term agreements with contract manufacturers.
Each product in Cytiva's BioProcess product portfolio has unique supply chain, quality and manufacturing needs. A tailored risk management approach helps to create more effective business continuity programs.
Between 2017 and 2022, Cytiva has been investing $70 million USD annually to significantly increase production capacity at its resin manufacturing site in Uppsala. In addition, Cytiva is investing $600+ million USD in establishing a new resins manufacturing site in the US. We also increased the security of supply ‘Strategic reserve’ stock of chromatography resins to more than 90 final products and covers approximately 6 to 12 months’ supply depending on the recovery plan for each product, until manufacturing is up and running according to our business continuity plan. This is Cytiva's own risk mitigation stock called a strategic reserve, which is stored separately from the Uppsala manufacturing site. It is to be used for the production of regulatory-approved biopharmaceuticals should an incident impact the site’s manufacturing capacity.
We also invest in research and development (R&D) activities to better understand and map critical quality attributes of raw materials that we use in the manufacturing of chromatography resins and cell culture media, and run replacement programs of Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) to prove like-for-like products post replacement and reduce variability in our chromatography products.
However, these efforts are not effective without transparent and regular communication across the supply chain. Sharing data and looking at the longer term to determine how suppliers and manufacturers can support each other creates a common ground, where both sides can act early in the face of supply chain challenges.
Security of supply strategies that encourage a continuous exchange of information allows the industry to see, understand, and ultimately have better control of its raw material supply, raw material variation, and the process impact of variability. Regular and transparent communication is vital to achieving this goal, and there are also a number of discussion forums that can be utilized, such as the Biophorum Operations Group forum.
Platforms such as this give stakeholders an opportunity to communicate with each other and obtain guidelines on how to drive these conversations with suppliers and regulatory bodies as well as amongst industry peers. This type of collaboration and transparency is the key to building an effective supply network and securing the supply of drugs to the patients who depend on us.
- Global Data database September 2021
- Evaluate Pharma. (July 2021).World Preview 2021, Outlook to 2026. Retrieved from https://info.evaluate.com/rs/607-YGS-364/images/WorldPreviewReport_Final_2021.pdf