Biopharmaceuticals have swiftly risen to great heights in the world of medicine, and it’s clear that they are here to stay. These life-saving drugs offer high efficacy and fewer side effects for many disease conditions and generated more than $275 billion last year in global revenue.1 But on the flipside, making these biological molecules is not simple – they are constantly evolving, with sophisticated structures that beget an equally complex set of operational and technological challenges. In tackling these challenges and achieving long-term manufacturing success, it’s important to establish an optimized production process and outcome.
In this article, we take a look at how the process outcome can be improved by increasing the performance and robustness of the bioprocess workflow through a variety of critical aspects, different strategic approaches, and innovative tools and methods. For a successful optimization, the teams managing the process must have a good understanding of its various elements and how they can be augmented to support an enhanced outcome. For example, things like streamlining operations, optimizing titers and yields, evaluating cell lines and cell culture media, and controlling raw material variability can play a pivotal role in obtaining a highly purified, safe, efficacious, and cost-effective product.
Streamlining process operations for a seamless workflow
It should be easy to move between each step in the process workflow, without having to make time-consuming adjustments. This means that connections should be optimized to reduce manual interaction and minimize human error – automating simple manual steps like adjusting pH, diluting/concentrating a sample, and transfer between vessels can help in this regard. Also, facilitating smooth technology transfer between process development and biomanufacturing will ensure minimal batch variability and a faster, continuous workflow from start to finish. In this way, streamlining operations can improve speed, robustness, efficiency, and consistency.
High titers vs. high yields
When evaluating the product output, titer and yield are two of the different measures used. Titer is the crude mass of protein in the total bioreactor liquid volume (measured typically as grams per liter), whereas the yield is the mass ratio of the final purified protein of interest to the total protein expressed.2 So, the titer characterizes the upstream bioprocessing efficiency, while the yield refers to the downstream efficiency. Although high titers generally indicate that a higher amount of the desired product is made, if the correct molecule is not efficiently expressed upstream and there are many impurities, the downstream yield will be low.
Figure 1. Inside a Cytiva plant with bioreactors
For instance, your titer might be 5 g/L, but if your yield is 50 percent, it’s not an efficient process. Particularly with respect to biosimilars, the charge variant profile of the product should closely match that of the originator molecule for regulatory approval. In most cases, although the titer is high, charge variant impurities can cause significant loss of yield during purification. On the other hand, a titer that’s 3 g/L may result in a higher quantity of the purified product, if the expression system is competent and the yield is high (>90 percent). As such, the critical aspect here is not the titer or the yield, but rather the correct expression of the desired molecule. To that end, having the best-suited cell lines and culture media that can minimize impurities and generate the correct product efficiently becomes important in improving process outcome.
Cell line and cell culture media are key
It’s common knowledge in the biopharma field that if you don’t start with the right cell line, you will never get a robust and efficient bioprocess – everything is built upon the cells, which are in fact the actual “factories” that synthesize the product. So, selecting a cell line capable of generating the biomolecule of interest at high yields and productivity is a vital first step in cell culture process development. Then, the cells should be supplemented with a high-performing cell culture medium, coupled with optimized bioreactor culture conditions and mode of operation. The result of these actions is optimal cell productivity and increased product quality/consistency at reduced operating cost.3
Figure 2. A scientist working on cell culture in the small scale
A platform approach supports rapid process development and robust outcome.
A common practice is to re-use know-how to make process development faster and more predictable by employing a platform approach. This is a great way to standardize drug development and manufacturing. A platform approach relies on a generalized or standardized platform and predefined protocols – it establishes a framework that can be tweaked a little for different molecules as needed, but essentially carries out the same generic steps. This approach is convenient for customers who need similar types of molecules to be produced and purified. With standard operating procedures and checklists, and generic equipment and raw material, the platform approach is robust for this purpose and ensures a certain level of performance (similar yield, purity, etc.) across the board for a certain range of molecules.
Quality by design and the need for deeper process understanding
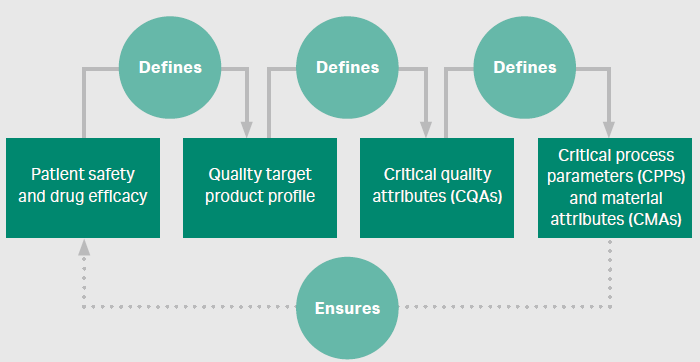
Regulatory authorities demand that manufacturers know their process inside out and anticipate all outcomes, regardless of how much time such evaluations take. Essentially, quality by design (QbD) is a complete chain of events that is driven by the outcome of satisfactory patient safety and drug efficacy and, retroactively, defines critical quality attributes and critical process parameters in manufacturing. The QbD approach helps integrate all aspects of product development – from the small to large scale continuous processing – to improve yields, while also maintaining the purity and safety of the final product. This involves a deep understanding of the process and how each step works, to generate a quality product.
Figure 3. A QbD approach to biopharma process development
The best way to enhance process outcome and ensure success in commercial manufacturing is to design a process that is robust, effective, and economical. For this, each step of the process must be carefully studied to identify target areas of improvement, especially as processes become larger and more costly in late-stage development. It is recommended to apply a risk-based strategy to process development, focusing on areas with the largest risk of failure. With increased molecular diversity, there is a heightened need to understand the interplay between critical process parameters (CPPs) and critical material attributes (CMAs).
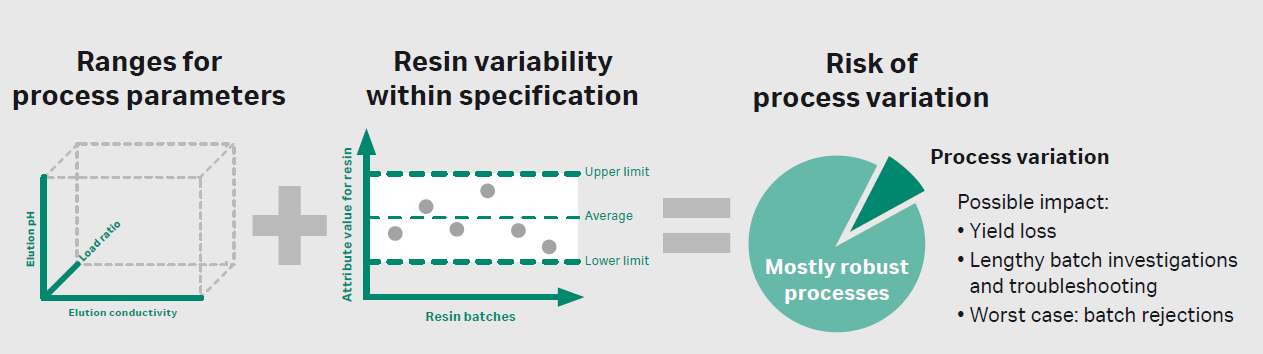
Raw materials, which may include anything from cell culture media to chemical additives to chromatography resins, might be a cause for manufacturing deviations. Variability of raw materials could have a significant impact on the quality and consistency of the final product, and often it may be difficult to track down the root cause at later stages of development.4 Issues due to variability can arise upstream in the cell culture or fermentation step, as well as downstream in purification. For example, chromatography resin variability might interplay with process parameters, (depending on the molecule) and lead to yield loss, lengthy batch investigations and troubleshooting, and even batch rejections. Manufacturers and suppliers must address these issues immediately to understand, monitor, and control raw material variability across the biopharmaceutical value chain – from raw material suppliers to patients.
Figure 4. The interplay between process parameters and resin attributes might lead to process variation
Modern tools and methodologies to improve purification processes
There are many tools and technologies available to help biopharma manufacturers achieve process robustness or enhanced outcomes. Some options that focus on enhancing purification methods for a better outcome are listed below:
- Process characterization kits
These kits allow for deeper chromatography process understanding, by helping study the potential impact that resin ligand density might have on the process outcome. With process characterization kits, manufacturers can get critical insights on resin variation and develop a solid control strategy to obtain a robust and scalable downstream chromatography process. - Validation Columns for viral clearance
Although they are an important step in the production of biologics, performing accurate and cost-effective viral clearance studies can be challenging. A good tip to help improve the robustness of these studies is using pre-packed, scale-down columns with a narrow inner diameter.5 Validation columns are designed as such, for reproducible process validation and process development. They provide high consistency between columns in terms of efficiency and asymmetry. Because of their dimensions – 10 mm inner diameter and 20 cm bed height typically used in biomanufacturing – these columns are especially suited for robust scale-down viral clearance studies. - Enhanced chromatographic resins
Some recently developed chromatography resins can help significantly in enhancing process outcome. For example, MabSelect PrismA protein A affinity resin, demonstrates improved alkaline stability and high dynamic binding capacity for purifying antibodies, including bispecific antibodies. Even under very harsh cleaning conditions (0.5-1.0 M sodium hydroxide) MabSelect PrismA is stable, with no effect on the resin capacity in the long term. This helps increase bioburden control for continuous processes, which can be exposed to days or weeks of feed harvest. - Mechanistic modeling in chromatography
Mechanistic modeling can help achieve better process outcomes and speed up process development by using computer simulations to decrease the number of chromatography experiments needed. For example, this methodology uses differential equations that describe how molecules move between resin beads and inside the bead pores. Mechanistic modeling has been heavily used in academic research, but now we are seeing this technique moving to the biopharma industry as well, to guide process characterization efforts and help scale up from lab scale columns to process columns.
Conclusion
Overall, it’s important to note that process understanding is the cornerstone of process development. In addition to balancing titer and purity and optimizing cell culture, manufacturers must study and understand how the interplay of process parameters and raw material variability can impact process outcome. In this way, process parameter targets can be defined and achieved to ensure a robust process performance.
Further reading
- Quality by design in biotherapeutic purification
- Robust scale-down models for chromatography process validation
- Cell Culture Titer & Protein Quality
- Optimization of fed‑batch culture conditions for a mAb‑producing CHO cell line
References:
1Morrow, K. J., Langer, E. S. A Biopharma Year In Review — And A Look Ahead To 2020. Biosimilar Development (2019).
2Rader, R. A., Langer, E. S. 30 Years of Upstream Productivity Improvements. BioProcess International (2015).
3Li, F., Vijayasankaran, N., Shen, A. et al. Cell culture processes for monoclonal antibody production. mAbs (2010), 2(5): 466–477.
4Chalk, S. Raw Material Variability. BioPharm International (2014), 27 (4).
5Troeng, L. Viral clearance: 7 chromatography column considerations. Cytiva Blog (2019)