Microbiological testing is critical in ensuring the quality of pharmaceutical products, as regulations become more stringent. With the regular discovery of new biologics, performing bioburden, sterility and microbial limit testing of raw materials, intermediates or finished products is a crucial step in the dynamic work done by pharmaceutical producers every day.
The membrane filter technique is a regulatory approved method for bioburden testing and microbial enumeration; Total Yeast and Mould Count (TYMC) and Total Aerobic Microbial Count (TAMC).
The Membrane Filtration (MF) technique is a well-accepted reliable method for fast, flexible testing, whether testing materials with concentrated or very low levels of microbes. Introduced in the late 1950s, the technique involves less preparation than many traditional methods and offers a number of advantages;
- Permits testing of large sample volumes
- Reduces preparation time as compared to many traditional methods
- Allows isolation and enumeration of discrete colonies of bacteria
- Provides presence or absence information within 24 hours
- Allows for removal of bacteriostatic or cidal agents that would not be removed in pour plate, spread plate, or most probable number (MPN) techniques
Maximizing efficiency and controlling contamination are paramount for microbiology applications. Cross-contamination during testing can result in false positives requiring costly retests and delaying product from reaching the market.
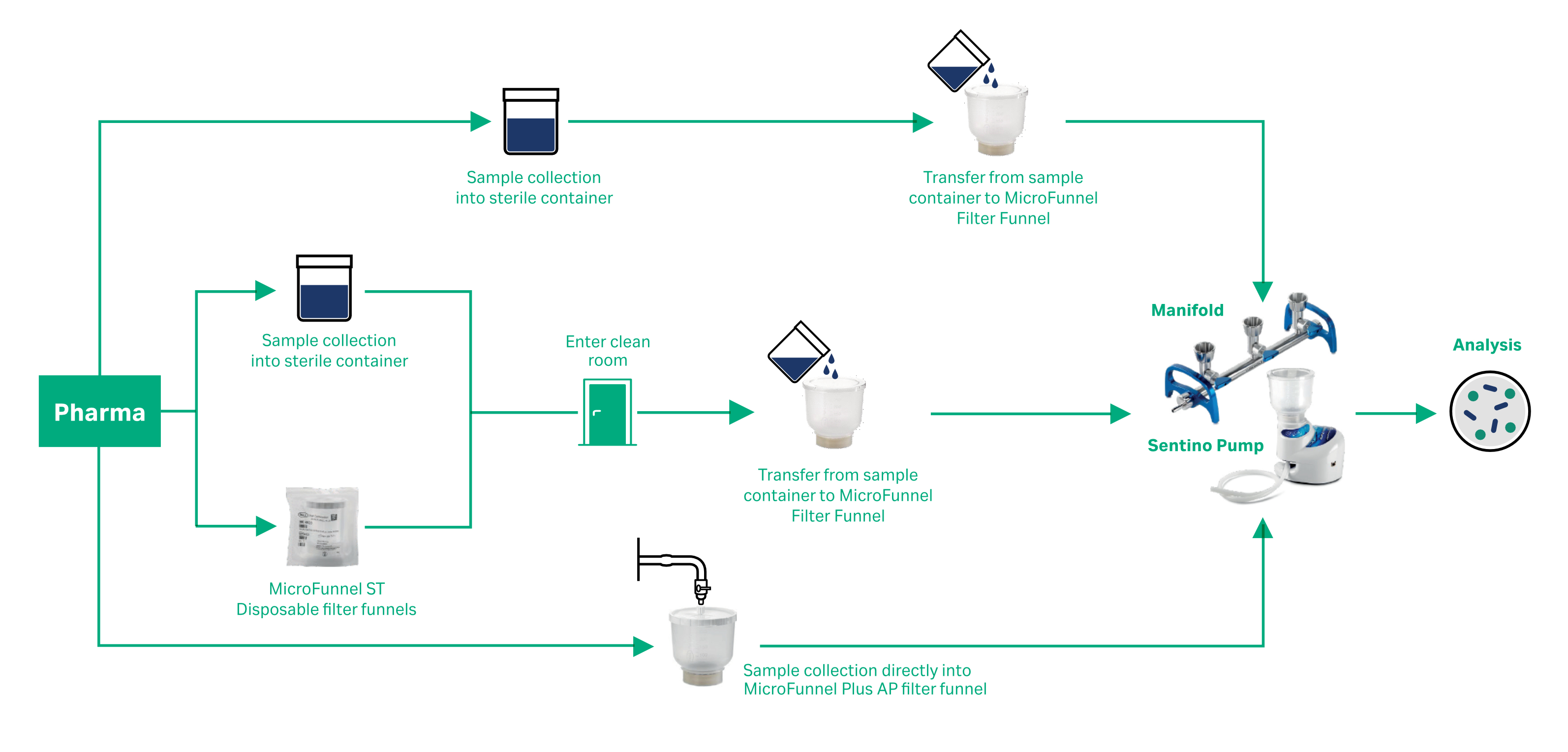
The MF technique can be performed by utilizing different laboratory workflows which could implement reusable hardware, partially disposable products, or individual disposable funnels.
Disposable, ready-to-use filtration systems are typically well-suited for quality control analysis of aqueous fluids used in pharmaceutical and biologic production, as they can limit cross-contamination.
Cytiva offers a suite of funnels, membranes, and equipment that is designed to be flexible for different pharmaceutical microbiology testing workflows.
MicroFunnel™ Filter Funnels
Disposable, gamma irradiated MicroFunnel filter funnels (Pall™ Life Sciences products) come ready-to-use, fully assembled, with both filter membrane and funnel. Featuring a convenient and ready to use design, these disposable filter funnels increase the productivity and efficiency of busy laboratories that do not have time to clean and sterilize reusable hardware.
Microfunnel filter funnel units provide an easy squeeze separation of the funnel from the base, preventing membrane disruption and offering fast, no-hassle operation. The base of the funnel can easily convert to a petri dish for culturing using the provided funnel lid. Alternatively, the membrane can be easily removed for placing on a separate agar dish.
The units are available in either 100 mL or 300 mL volume sizes with either 0.2 µm, 0.45 µm, or 0.8 µm membranes. Each lot is certified for microbiological analysis to provide added assurance of reliable results. They are individually bagged, eliminating potential cross-contamination risks.
A double-bagged version, MicroFunnel ST filter funnel, is available which saves time on entry into cleanrooms, isolators or hoods with only one surface to spray down.
MicroFunnel™ Filter Funnels
MicroFunnel™ Plus Filter Funnels
MicroFunnel™ Plus AP Filter Funnels
MicroFunnel Plus filter funnel units have further evolved the design of the MicroFunnel filter funnels in order to improve efficiency and offer increased protection from contamination. The MicroFunnel Plus filter funnels feature a vented lid that snaps closed to ensure a liquid-tight seal. This allows for sample collection, transportation, and filtration all in the same unit.
A separate sample cup is no longer necessary for collection, and there is no need to transfer your sample into a disposable funnel for concentration. The vented filter on the lid prevents airborne contamination that could be drawn into the funnel during filtration.
The MicroFunnel Plus AP unit allows for the aseptic collection of a sample through the lid sample port on the filter funnel, without the need to remove the lid.
Microbiology Manifold and Sentino™ Pump (Pall™ Life Sciences products)
When performing pharmaceutical microbiology limiting cross-contamination is crucial. We provide microbiology manifolds and pumps that can be used in tandem with Microfunnel filter units and have design features to reduce the risk of cross-contamination.
The microbiology manifold allows you to optimize testing without sacrificing cleanliness.
- Requires no tools or tape to put together
- Disassembles easily for thorough cleaning
- Inlet and outlet can be set up at either end of the manifold, avoiding the risk of contamination due to reaching over the top of funnels to turn on and off valves
The manifolds are available in a 3-place format, which can eaily be coupled together to form a 6-place manifold. The modular design allows for easily separation for disinfection and/or sterilization.
Microbiology Manifold
Coupled Microbiology Manifolds
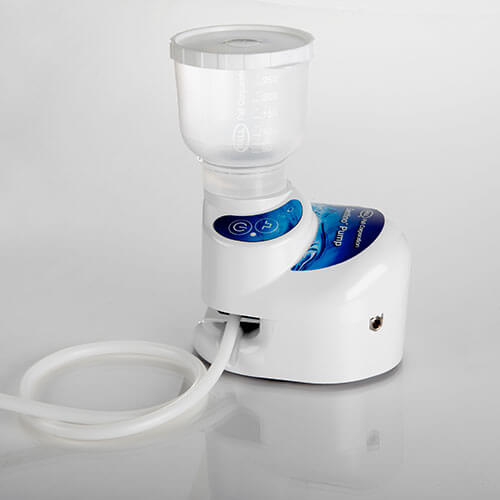
Sentino™ Microbiology Pump
The Sentino Pump streamlines analysis by replacing the traditional vacuum filtration system with a small peristaltic-action pump that draws samples through MicroFunnel filter funnel units. The filtrate is channeled directly to drain or waste collection through a disposable fluid path. The fluid path is simple to load and completely disposable to eliminate potential biofilm build-up. There is no need to clean, wrap, and autoclave a bulky multi-place manifold. The potential for sporadic and unnecessary contamination is removed.
The unit’s compact design makes it easy to use in confined spaces and frees valuable benchtop space. It also provides flexibility in arranging workspace for optimal efficiency and workflow.
A soft-touch keypad featuring simple on/off and pulse functions makes it easy to operate, with no complicated programming to validate. Operating parameters are preset and fixed to meet the published requirements for Membrane Filtration (MF) Technique as described in ISO and ASTM methods, thereby eliminating the need for extensive validations.
When performing pharmaceutical microbiological testing as well as limiting cross-contamination it is also important to select high-performance products with ergonomics
The repetitive nature of routine laboratory work puts pharmaceutical technicians and scientists at risk for repetitive strain injuries (RSI). Routine activities often include repeat of the same movements over and over, which can take a toll on hands, wrists, and shoulders and can lead to serious injury. RSI can lead to laboratory user fatigue and variability that causes poor technique, errors, and increases cross-contamination.
In a pharmaceutical microbiology laboratory, there are many ways to avoid manual labor stress by working in a more ergonomic manner. Choosing the right tools can make all the difference.
Download our Scientific Brief - How Ergonomics and Cleaning Ease Reduce Repetitive Stress Injuries and Contamination in Pharmaceutical Lab Workflows