- Email notifications when a new CCN is published
- Access to history of all previously published CCNs for a specific product
- Possibility to download files in pdf format
- Possibility to use a generic company email address (recommended)
- Download CCN service flyer
Bioprocessing
- Cell Culture Media
- Instruments
- Consumables
- Software
- Instruments
- Consumables
- Software
- Instruments
- Consumables
- Software
Link to Standard for Cytiva Change Control Process for Designated Products
Changes for products that are handled according to our change control process are documented and can be tracked. We always perform a risk or impact analysis, as well as consider validation, when changes are made. The result of this analysis will determine if a CCN is published. Certain types of changes are always communicated via a CCN.
Some examples of changes that may be subject to a customer notification:
- Change of manufacturing site
- Change of raw material
- Change of specification limit within/outside current limits
- Change of label and/or packaging material
- Change of critical subcontractor
This process applies to products covered by the Standard for Cytiva change control process for designated products.
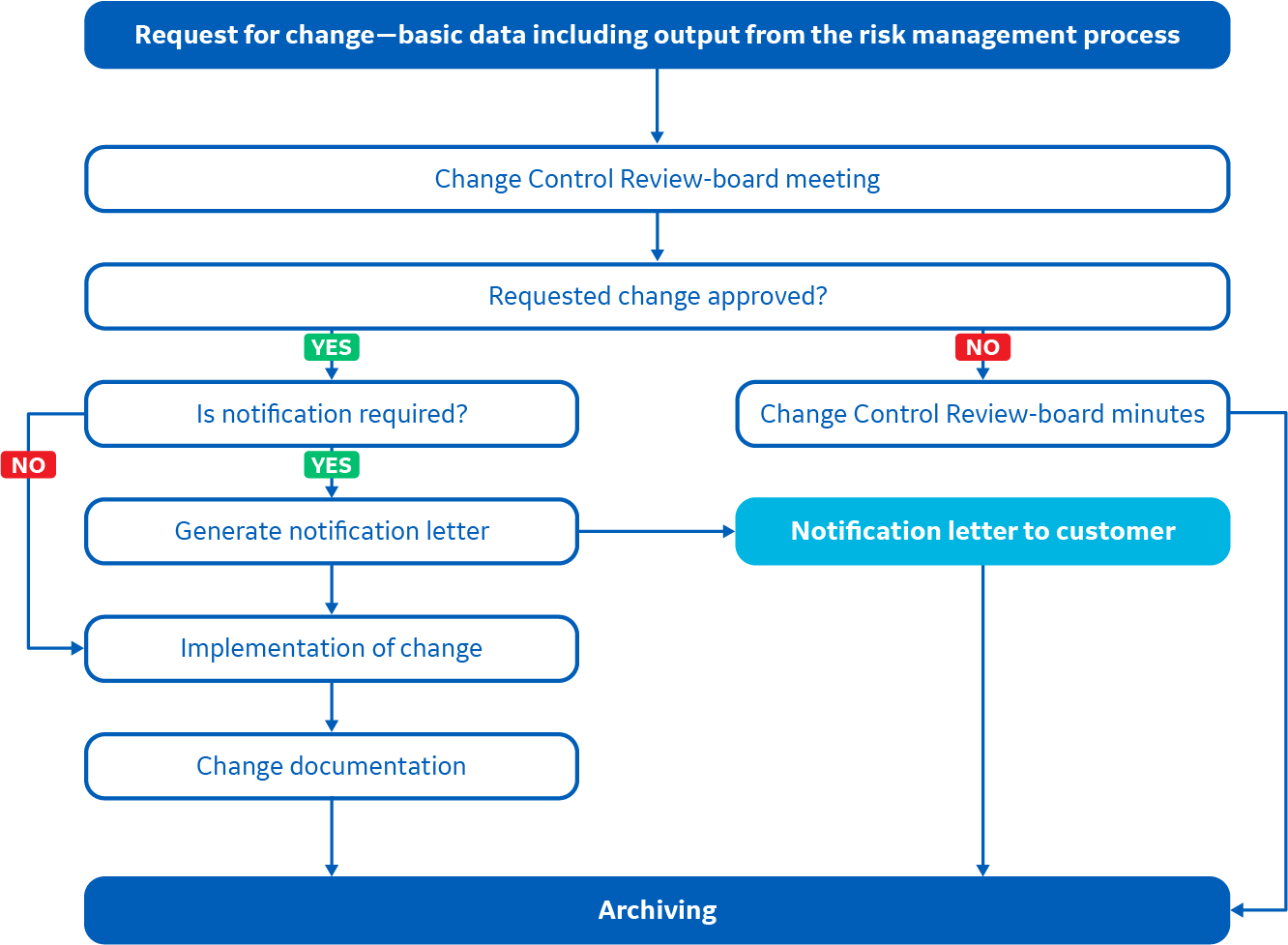
This process workflow applies to products covered by the Standard for Cytiva change control process for designated products.