Multidimensional scale-up of a monoclonal antibody capture step
Fast and efficient process development and scale-up contributes to a shortened time to market. Due to the convenience, time, and cost; bioprocess development is preferably conducted in small scale. The subsequent scale-up is performed in one or more steps, usually 20- to 30-fold per step, depending on the final production scale.
Equipment that can be adapted to the different stages of the development process facilitates this work - equipment that provides simplified bioprocessing, a high level of automation, and compliance with GMP is required to ensure quality of the bioproduct.
Introduction
In this work, a multidimensional scale-up (change of both column diameter and bed height) of a mAb capture step, using the ÄKTA pilot 600 chromatography system, is demonstrated.
Column volume per hour (CV/h) was utilized to enable maintaining residence time throughout the scaling process. The process, performed with MabSelect PrismA Protein A chromatography resin, was scaled up 20-fold from a 70 mL HiScale26 column to a 1400 mL AxiChrom 100 column. Equivalent mAb recovery and purity was achieved between the larger and the smaller scales, showing a robust and scalable process.
Fig 1. ÄKTA pilot 600 bench-top chromatography system can be used in both GMP (“regulatory“ version) and non-GMP (“standard” version) environments.
Results from the mAb capture scale-up study
Method transfer from ÄKTA avant 150 to ÄKTA pilot 600
The process was transferred to ÄKTA pilot 600 by creating a new method using the same volumetric flow and volumes for each process stage as used in the smaller scale on ÄKTA avant 150. The wide flow rate range of ÄKTA pilot 600 allowed testing the process in the HiScale 26 column to detect possible need for method modifications without wasting a large amount of sample.
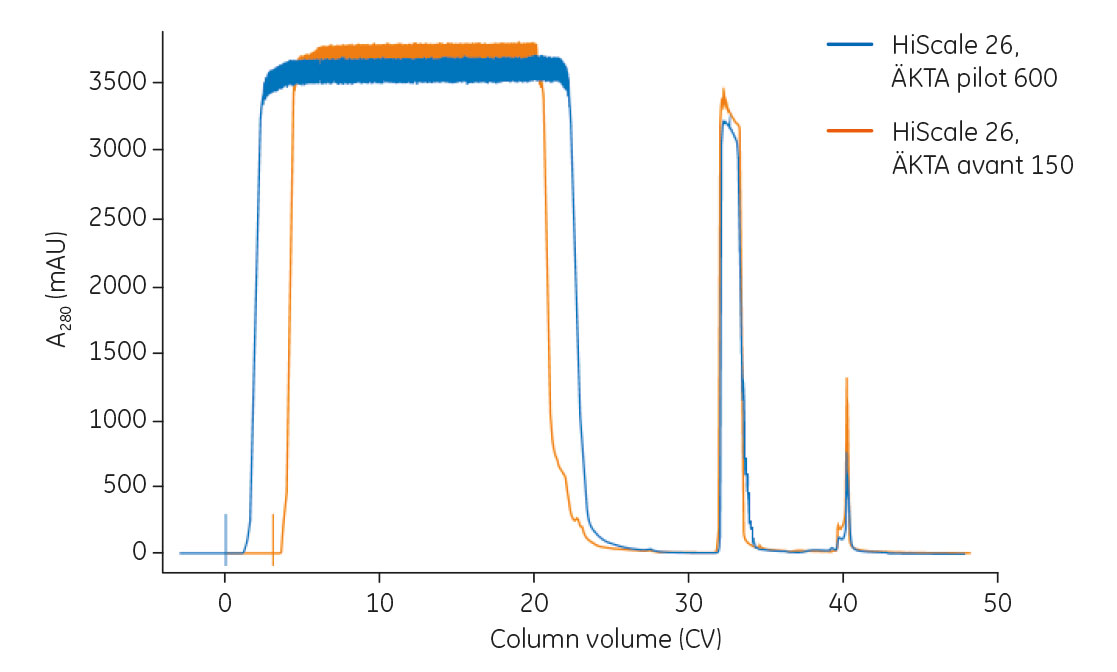
An overlay of chromatograms from the process run on ÄKTA avant 150 as well as on ÄKTA pilot 600 is shown in Figure 2.
Fig 2.Overlay of chromatograms from mAb capture on the HiScale 26 column using ÄKTA avant 150 and ÄKTA pilot 600. The two curves are aligned from the start of elution. Feed concentrations were 3.5 mg/mL for the ÄKTA avant 150 process and 2.7 mg/mL for the ÄKTA pilot 600 process.
The system hold-up volume (in CV) can be seen as the delay from the start of the sample load until the UV signal starts to increase. The difference at the end of the flowthrough is because the feed in the run performed on ÄKTA avant 150 was manually chased with equilibration buffer (i.e., added to the sample feed at the end of loading to ensure all sample was loaded).
Scale-up on ÄKTA pilot 600
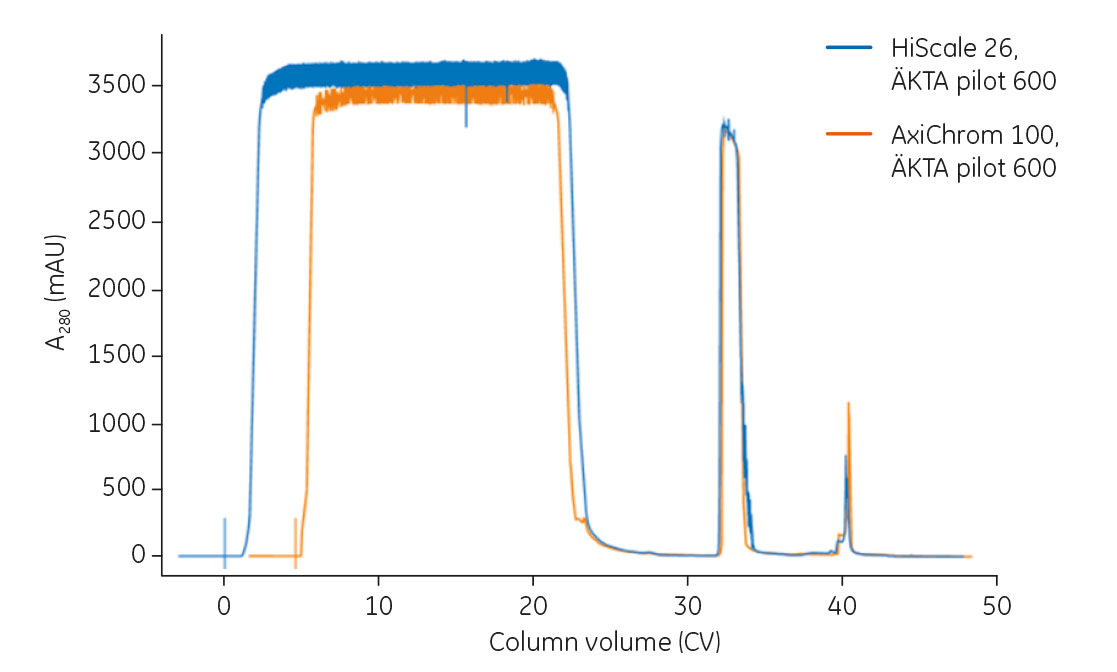
When scaling using the flow rate unit CV/h, the method can easily be changed in Method settings of the software by changing column type, and the flow rate will be automatically adjusted for the new column volume. An overlay of chromatograms from the process run on HiScale 26 as well as on AxiChrom 100 using ÄKTA pilot 600 is shown in Figure 3.
Fig 3. Overlay of chromatograms from mAb capture on the HiScale 26 and AxiChrom 100 columns, both operated on ÄKTA pilot 600. Feed concentrations were 2.7 mg/mL for the HiScale 26 column and 3.0 mg/mL for the AxiChrom 100 column.
The observed difference in the flowthrough is due to the use of different feed concentrations and that the sample in the larger scale was manually chased with equilibration buffer.
Modifications to a GMP-regulated environment
In a regulated environment, it is necessary to have cleaning procedures in place for both the system and for system components. To simplify operations, it is beneficial if the system can be configured with only the components needed for the process (e.g., number of inlets and outlets, and with or without mixer).
ÄKTA pilot 600 can be configured for use in process development (“standard” version) as well as in small-scale GMP production (“regulatory“ version), where its high level of automation ensures a robust production process.
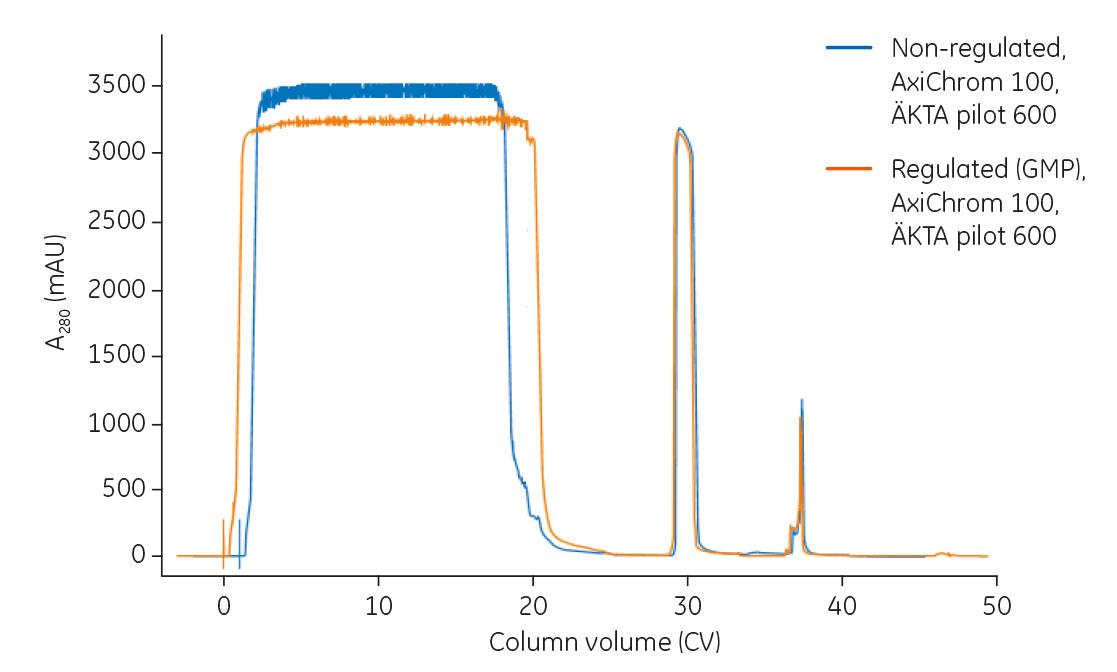
Once the scaled-up method was established in ÄKTA pilot 600, the method was modified to fit GMP production. An overlay of chromatograms from the process run on ÄKTA pilot 600, configured for a non-regulated as well as a regulated environment, is shown in Figure 4. The observed difference in the flowthrough is due to the difference in feed concentration between the runs.
Fig 4. Overlay of chromatograms from mAb capture on AxiChrom 100 using ÄKTA pilot 600 in a non-regulated (non-GMP) and regulated (GMP) environment. Feed concentrations were 3.5 mg/mL for the non-regulated process and 3.0 mg/mL for the regulated process.
Analytical results
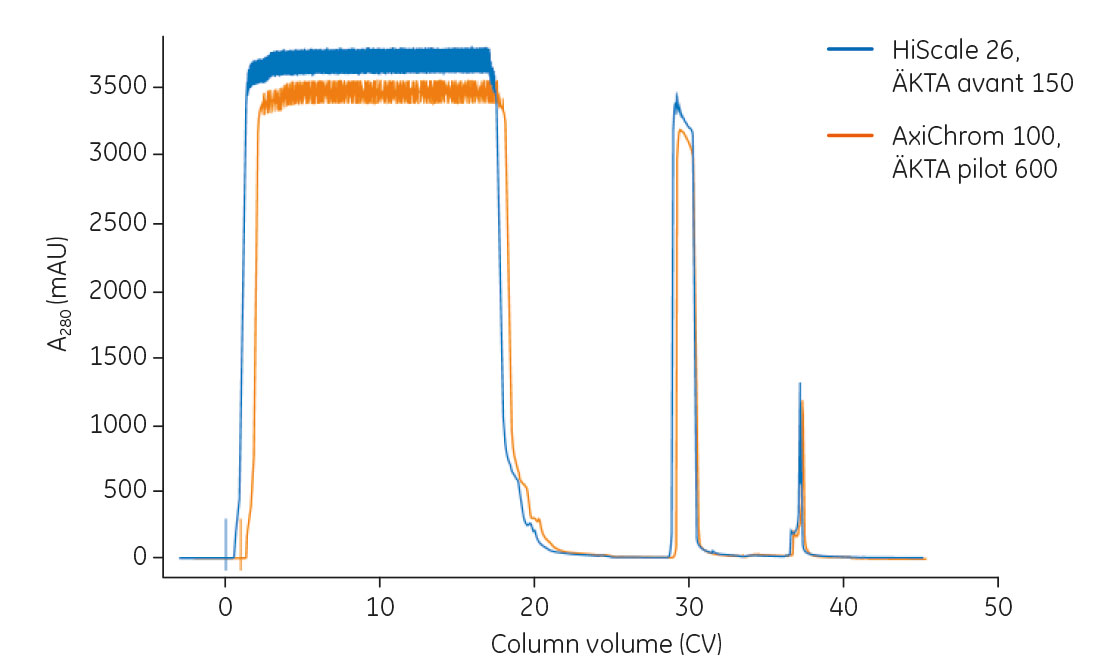
An overlay of chromatograms from mAb capture on the HiScale 26 column using ÄKTA avant 150 and on the AxiChrom 100 column using ÄKTA pilot 600 is shown in Figure 5. mAb recovery and purity from duplicate runs in both setups are summarized in Table 1.
Fig 5. Overlay of chromatograms from mAb capture on the HiScale 26 column using ÄKTA avant 150 and on the AxiChrom 100 column using ÄKTA pilot 600.
Table 1. Recovery and impurity removal on the HiScale 26 column using ÄKTA avant 150 and on the AxiChrom 100 column using ÄKTA pilot 600
| Column | Column volume (mL) | mAb load (g) | Recovery by UV, A280 (%) | Recovery by SPR (%) | Aggregates (%) | HCP (ppm) | Protein A (ppm) |
| Feed | 173679-184428 | ||||||
| HiScale 1 | 70 | 4.2 | 95.0 | 96.8 | 1.14 | 256 | 17 |
| HiScale 2 | 70 | 4.2 | 103.0 | 98.7 | 1.48 | 295 | 12 |
| AxiChrom 1 | 1400 | 82 | 95.8 | 105.9 | 1.02 | 300 | 13 |
| AxiChrom 2 | 1400 | 82 | 104.4 | 97.4 | 1.03 | 292 | 8 |
Conclusion
A 20-fold multidimensional scale-up was performed, starting from the small-scale process run on the HiScale 26 column operated on ÄKTA avant 150 and scaling up to the AxiChrom 100 column operated on ÄKTA pilot 600. Initially, ÄKTA pilot 600 was configured for process development for verification of consistency between processes run in the small and larger scales.
At this stage, the process was performed in a more manual workflow, and the system was configured with extra outlets for collection of flowthrough for subsequent analysis. Similar results in terms of mAb recovery and purity between scales indicate a successful scale-up from HiScale 26 to AxiChrom 100. The process was further modified to allow an automated workflow suitable for production in a regulated (GMP) environment.
This work demonstrates the use of ÄKTA pilot 600 from fast and efficient process development and scale-up (“standard” version) to production in a regulated environment (“regulatory“ version). The use of the same software in ÄKTA pilot 600 simplified method transfer from the smaller ÄKTA avant 150 system. Column volume per hour (CV/h) was utilized to enable maintaining residence time throughout the scaling process.
Download the multidimensional scale-up application note to get more details on this study.