Cell technologies for improved therapies
The most innovative studies in the field of biomedical research are presented at the meetings of the European Society for Animal Cell Technology (ESACT). In 2017, we revealed the results of some of the cell culture work we have been involved in to improve bioprocess efficiencies.
Discover the strategies and techniques used for cell culture medium development and optimization and fed-batch and perfusion processes by downloading and reviewing our posters.
See the results from our cell culture studies
Repurposing fed-batch media and feeds for highly productive cho cell perfusion processes
We have developed a method for identifying first-generation perfusion culture media based on existing fed-batch media and feeds. We show that we can obtain culture media that successfully support perfusion cultures in bioreactors at cell-specific perfusion rates below 25 pL/cell/day. High productivities and favorable product quality were also achieved.
Authors: Marcel Kuiper1, Eric Fäldt2, Audrey Vuillemez1, William Holmes1, Teres Persson2, David Gruber1, and Andreas Castan2
1 MedImmune, Biopharmaceutical Development, Cambridge, UK
2 Cytiva Bio-Sciences AB, Uppsala, Sweden
Download the poster:Repurposing fed-batch media and feeds for highly productive CHO cell perfusion processes
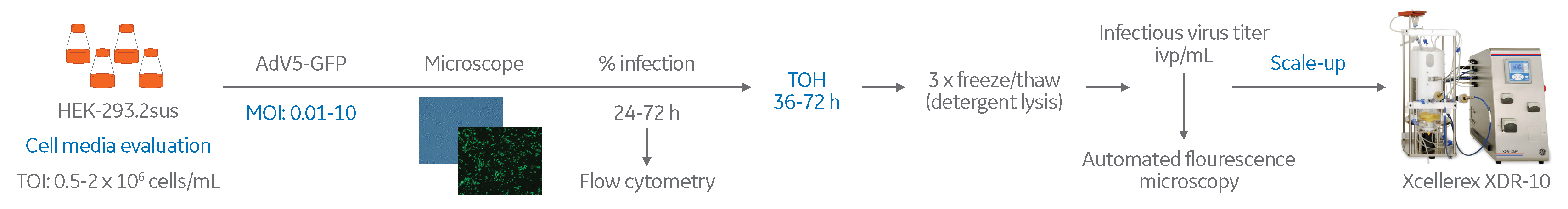
Adenovirus production in a single-use stirred-tank bioreactor system
Adenovirus vectors are attractive delivery systems for vaccines and cancer treatment. Scalable and cost-efficient production technologies are needed to enable manufacturing of safe and efficacious clinical-grade viruses. Anchorage-dependent cells cultured in roller bottles or cell factories are commonly used in these processes. However, scaling up using these techniques is complicated and limited by the surface available for cell growth. One alternative is to scale up the production on microcarriers. Another solution is to use suspension-adapted cells, which can facilitate scale-up possibilities.
In this work, we demonstrate an efficient process for adenovirus production in a single-use stirred-tank bioreactor, using HEK293 cells adapted for suspension culture. Human adenovirus 5 expressing green fluorescent protein (GFP) was used as a model system. By evaluating different cell culture media (CCM) and optimizing the virus propagation in small scale, the process could be successfully established in an Xcellerex XDR-10 bioreactor system (10 L working volume). This process opens up possibilities for further scale-up to production scale. The following strategy was used for this study:
Authors: Teres Persson, Gustaf Ahlén, Susanne Stier, Mia Bennemo, and Mats Lundgren
Cytiva Bio-Sciences, Uppsala, Sweden
Download the poster:Adenovirus production in a single-use stirred-tank bioreactor system
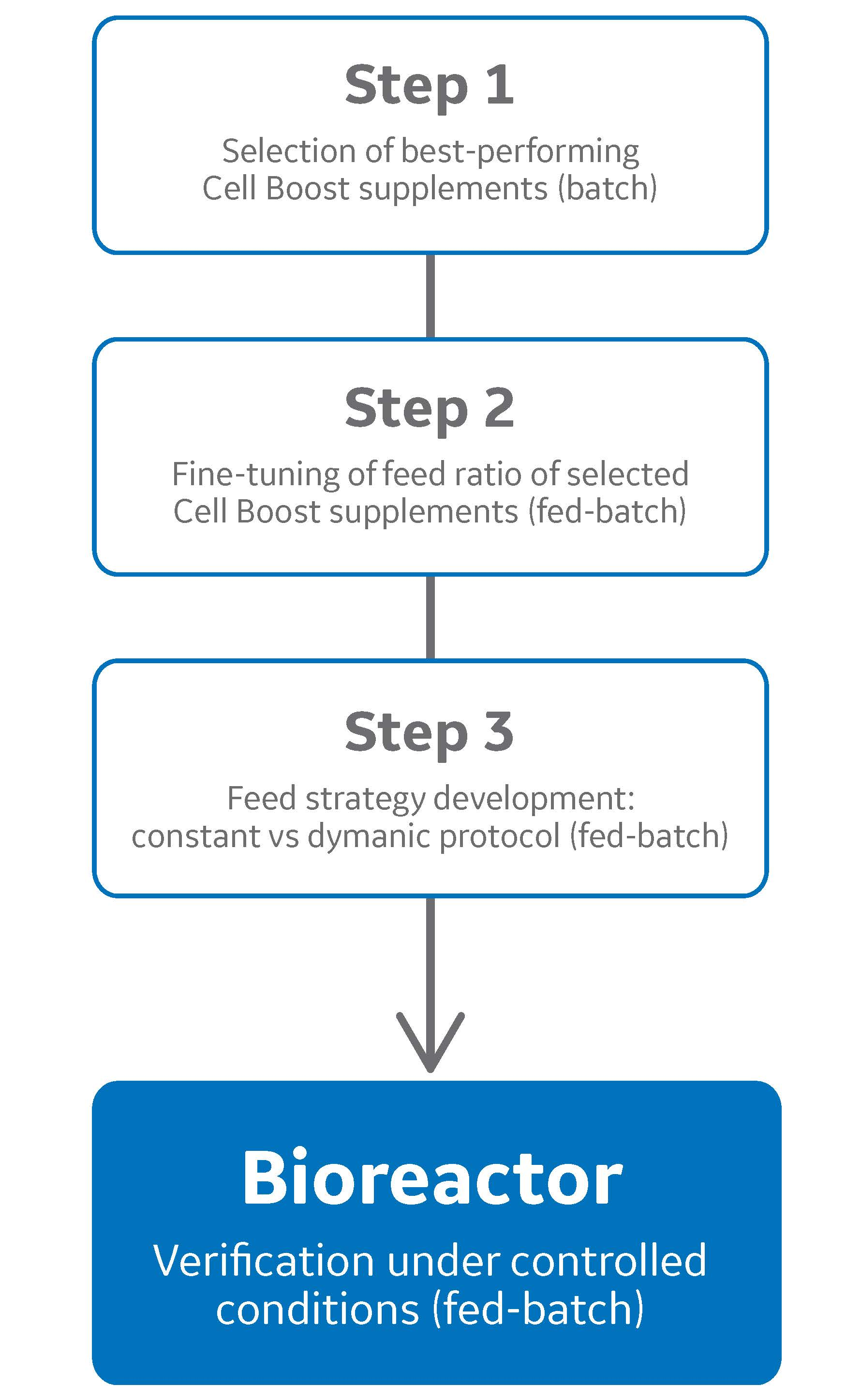
Development of doe-based fed-batch strategies for high-producing cho cell cultures
Fed-batch culture is commonly employed to maximize cell and product concentrations in upstream mammalian cell culture processes. Typical standard platform processes rely on fixed-volume bolus feeding of concentrated supplements at regular intervals. However, such static approaches might result in over- or underfeeding.
To mimic more closely the dynamics of a fed-batch culture, we developed a dynamic feeding strategy responsive to the actual nutrient needs of a mAb-producing recombinant Chinese hamster ovary (CHO) cell line.
Authors: David Reinhart1, Andreas Castan2, Lukas Damjanovic1, Barbara Holub2, Renate Kunert1
1 Vienna Institute of BioTechnology, Department of Biotechnology, University of Natural Resources and Life Sciences, Vienna, Austria
2 Cytiva Bio-Sciences AB, Uppsala, Sweden
Download the poster:Development of DoE-based fed-batch strategies for high-producing CHO cell cultures
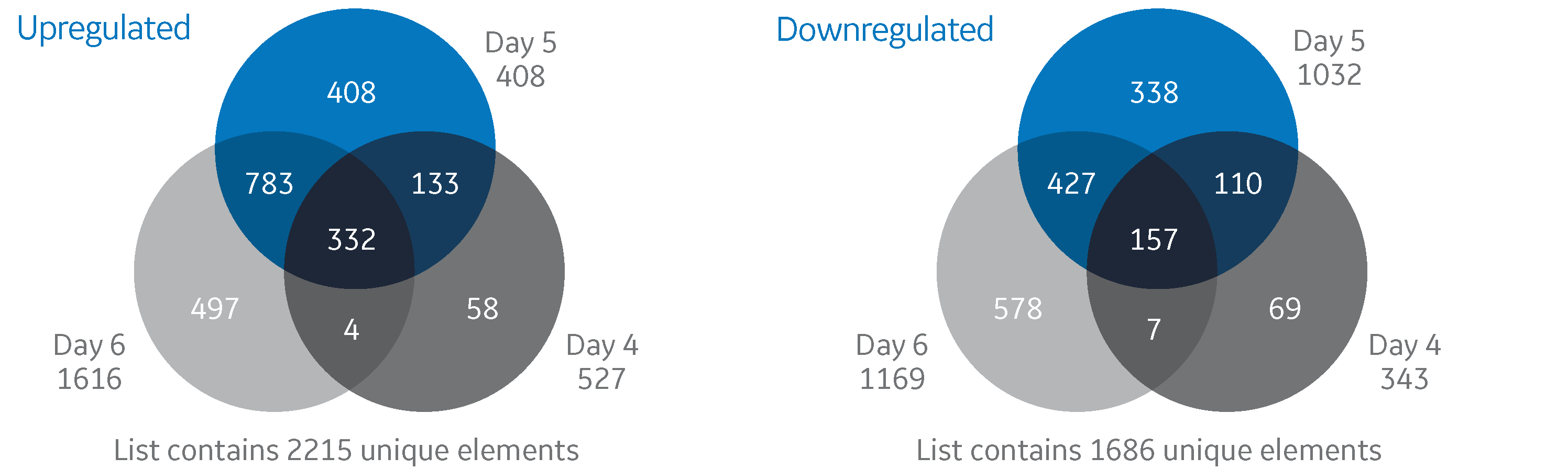
Transcriptome analysis in high-producing cho cell cultures: strategies to design high-performing cell culture media
The present study investigates the beneficial effect of spiking HyClone ActiPro basal medium with HyClone Cell Boost 7a and Cell Boost 7b feed supplements on growth and productivity of a recombinant Chinese hamster ovary (CHO) cell line. To evaluate the impact of feed-spiking compared with cultivation in basal medium only, the cell line was grown in bioreactors under controlled conditions to determine cell-specific metabolic rates, nutrient consumption, and byproduct accumulation over the process time.
Transcriptome analysis of the cultivated cells, using microarrays on four consecutive days to investigate differential gene expression, revealed the beneficial effect of feed-spiking compared with cells grown in basal medium. Differential gene expression revealed genes that appear important for high cell-specific production rates, and this knowledge can be leveraged into cell line engineering approaches or the design of media supporting high productivity in CHO cells.
Authors: David Reinhart1, Andreas Castan2, Lukas Damjanovic1, Wolfgang Ernst1, and Renate Kunert1
1 Vienna Institute of BioTechnology, Department of Biotechnology, University of Natural Resources and Life Sciences, Vienna, Austria
2 Cytiva Bio-Sciences AB, Uppsala, Sweden
Download the poster:Transcriptome analysis in high-producing CHO cell cultures: Strategies to design high-performing cell culture media
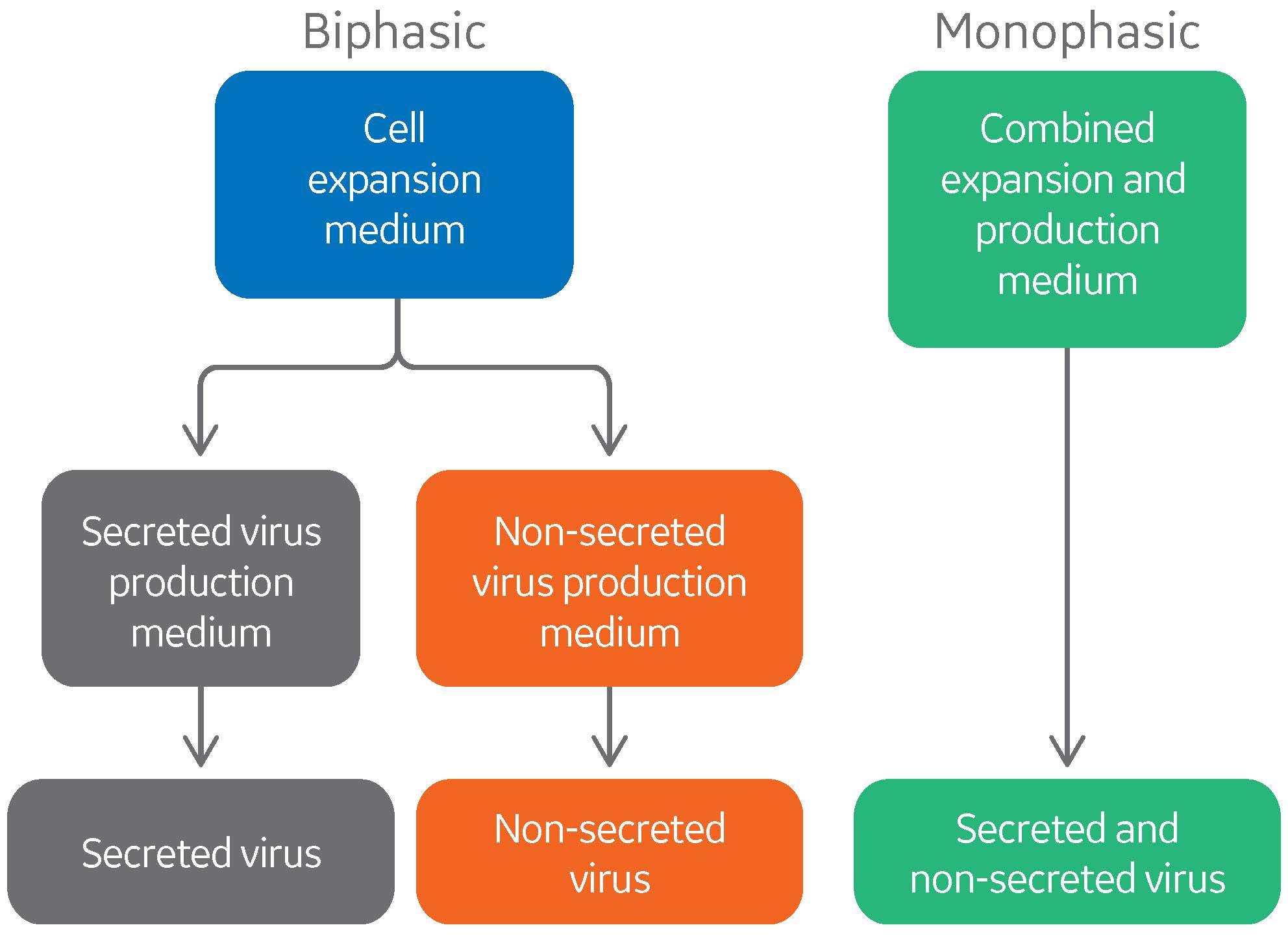
Development of chemically-defined medium for virus vaccine production in a duck suspension cell line
A project was initiated to improve the production process for viral vaccines through the use of the EB66® cell line derived from duck embryonic stem cells and proprietary to Valneva. The primary goal of this project was to develop a chemically defined medium that could be used in a simplified process, while supporting the growth and production of a broad spectrum of different viruses.
Many cell lines employed in vaccine production are obligate attachment cells, requiring non-defined additives or even serum to maintain successful culture conditions. However, EB66 cells grow in serum-free suspension culture at high cell density, allowing for much easier and more efficient scale-up, as well as virus production levels comparable to attachment cells.
As with many viral cell culture-based production processes, viral production in EB66 cells requires separate media for the cell proliferation phase and the virus production phase. Hence, the process includes two different basal medium formulations, with several separate additives or feeds required. While these processes are serum-free, they are not considered chemically defined.
Authors: John Manwaring1, Jeron Larsen1, Michael Sharp1, Mark Wight1, Françoise Aubrit2, Thomas Mollet2,
Sylvana de Clery2, Annabelle Landron2, Fabienne Guéhenneux2, Arnaud Léon2, Klaus Schwamborn2
1 Cytiva Bio-Sciences AB, Uppsala, Sweden
2 Valneva SE, Campus Bio-Ouest, Saint-Herblain, France
Download the poster:Development of chemically defined medium for virus vaccine production in a duck suspension cell line
- Simplifying cell culture expansion – Find out how we achieved a one-step seed culture expansion from cell bank to 2000 L bioreactor, lowering risk and cutting costs.
- How to achieve high concentrations of target molecules through efficient filtration – See how we achieved a concentration of an IgG antibody from 50.0 to 0.165 L in under 5 h, using a detailed method you can follow or adapt as needed.
- Smoother, simpler, smarter: how new technologies improved influenza virus production – Influenza virus production using the ReadyToProcess WAVE 25 bioreactor system and ready-to-use microcarriers. We took an established influenza virus production method and adapted it for use with our newly evolved product lines. Find out how the results compared with the original process.