Stepping up concentrations—a best practice example
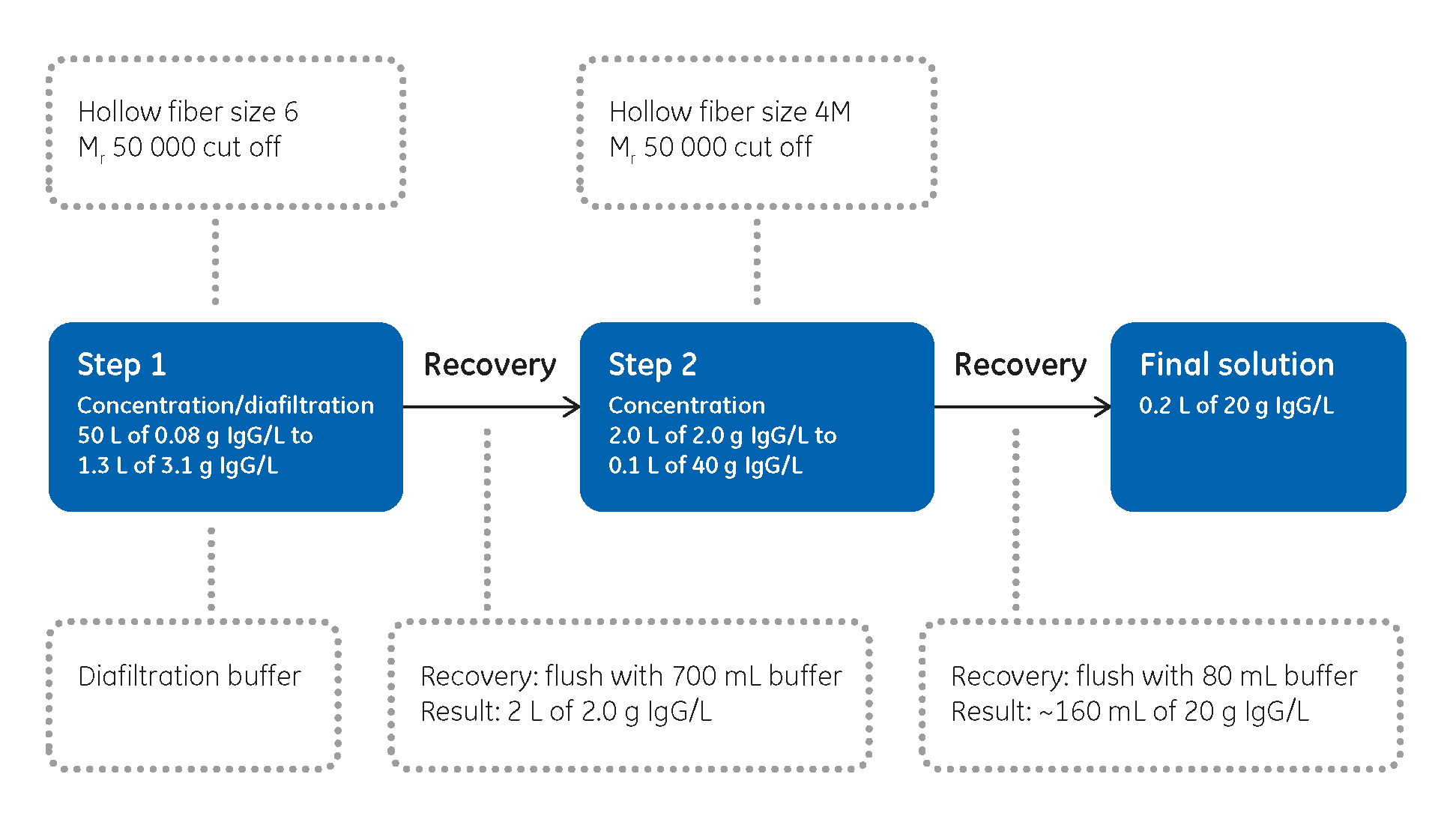
Knowing how to efficiently step up concentrations of target molecules to enable effective processing is essential in production processes for clinical trials. Understanding the processes and creating an optimized design for any filtration setup is a crucial first step. To help you, we have concentrated bovine IgG antibody from 50.0 to 0.165 L in less than 5 h. See how we achieved this in a two-step filtration process.
The scenario and results
Our goal was to concentrate a target molecule 500 times in the shortest possible process time. We looked at ways to optimize the design of circuits with low working volume that would allow for high concentration of dilute feed streams.
We achieved a concentration of 650 times with a total recovery of 100%. The two-step process times were 2.3 h and 1.9 h, respectively, for a total processing time of 4.2 h. Time for assembly for the two circuits was approx. 30 min per circuit, including setup and connectivity. Disassembly and disposal time for the these circuits were determined to be approximately 10 min per circuit.
Equipment used
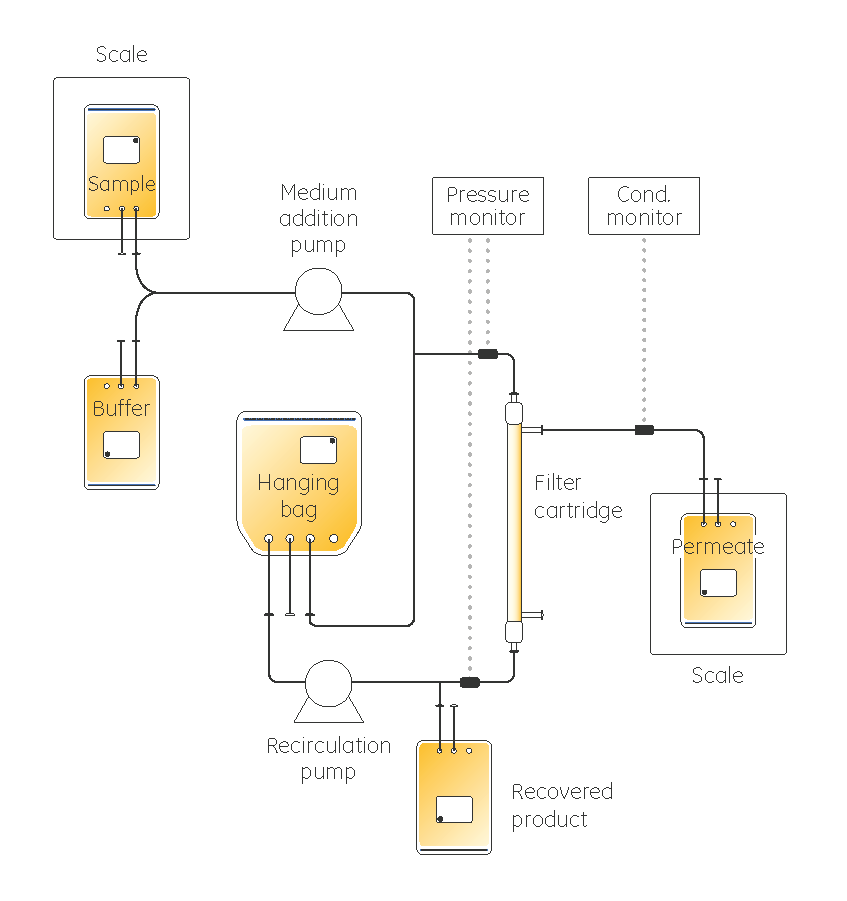
We opted for a closed system to minimize recirculation and hold-up volumes. To concentrate dilute feed streams up to 500 times, being able to use the system flexibly but with close control of the hold-up volume is essential. ReadyCircuit disposable assemblies fulfil these requirements and were used to design the circuits.
A low-titer IgG antibody sample was used as starting material.
Filtration was performed using ReadyToProcess hollow fiber filter cartridges. These filters are available in a wide range of base unit configurations and are readily integrated into single-use assemblies. See full circuit design details for both steps in our application note.
Why single-use hollow fiber filters over cassette filters?
Hollow fiber filters are especially suitable where the process stream needs to be contained for health and safety reasons. Cassette filters, on the other hand, often require user installation into a manifold assembly. The cassette and manifold assembly must be decontaminated during removal and disposal to prevent user exposure to potentially harmful process fluids. When using single-use assemblies, all process components that have been in contact with the process material, including the hollow fiber filter, can be conveniently disposed of after use, without the need for open handling of the product. In addition, single-use technology has the potential to offer time-savings in the end-to-end process through the elimination of post-process cleaning.
Setup and operation
Commercially available bovine IgG was dissolved in phosphate buffered saline (PBS), pH 7.4. To remove any particles, the IgG containing solution was filtered through a 0.2 μm filter before the concentration step. Thereafter, we used the following two-step process:
- Concentration and diafiltration
- Concentration
For the first step, a recirculation process with a calibrated pump was created, with great care being taken over flow rates and pressures. The process was designed as a fed-batch operation, where the liquid line in the reservoir was maintained at a constant level below the exhaust air filter line on the reservoir bag until all the dilute IgG feedstock was dispensed into the system. Thereafter, the medium addition pump was stopped and the concentration process was allowed to continue until the target sample volume was achieved.
During diafiltration, the fluid addition and permeate flow rates were matched to keep the sample volume constant. Techniques including a careful final flush and ways of recovering remaining material from the circuit were used to help maximize results.
For the second step, a fed-batch operation was again used, this time with smaller hollow fiber filters. Again, great care was taken over flow rates and pressures and how the final flush was carried out.
See full details of both steps in our application note.
How we achieved optimal results
The filtration circuits were specially designed with lowest possible working volumes to achieve a high concentration factor. Important for such a highly concentrated product recovery is the low point drain. For full recovery, air was forced into the system to allow complete emptying of the circuit. The successful use of stand-alone pumps and pressure sensors for process operations demonstrates flexibility in system use. The use of ReadyCircuit assemblies with ReadyMate single-use connectors enabled aseptic connectivity of the filtration device.
How you can produce equivalent results
You can use our process as a starting point. All the details including pressures, flow rates, timings and circuit diagrams are set out in full in our application note.
You will need to keep in mind that when concentrating larger targets, such as viral vaccines or virus-like particles, membranes of larger pore sizes might be required, resulting in a higher flux and a shorter process time. If you use the process as recommended, you should be able to concentrate your target, whether it is a viral vaccine or protein product, for final fill activities and clinical trial applications.
- If you are working downstream, find out how you can cut costs and minimize bottlenecks with our whitepaper - Unlocking the potential for efficiency in downstream bioprocessing.
- Find out more about the filtration expertise we offer in our brochure - Your needs, our capabilities.