Learn from fellow scientists
Development of efficient manufacturing processes is a necessity for cost-effective production of biopharmaceuticals. By carefully designing and evaluating your experiments, sufficient information for optimizing your process can be obtained with a minimal number of experiments. However, process development still demands a large number of experimental runs. Features such as easy method development, reliable unattended operation, and quick process scaling can help save time and optimize your equipment utilization. From this article, learn how representatives from the biopharmaceutical industry utilize ÄKTA avant chromatography system to gain efficiency in their development of purification processes for biopharmaceuticals.
INTRODUCTION
Process development demands rigor and can be time-consuming. Many experiments are required to generate sufficient information for an optimized design of your process. Studies are usually long, many times exceeding one working day. ÄKTA avant is a chromatography system designed to meet the market’s needs for efficiency in process development (Fig 1). The system is available in two versions: ÄKTA avant 25, designed for screening of chromatography resins and process optimization; and ÄKTA avant 150, suitable for scaling up to larger columns. ÄKTA avant is operated through the UNICORN system control software. The software has an integrated design of experiments (DoE) functionality to facilitate process development. For optimized equipment utilization during process development, ÄKTA avant exhibits a high degree of automation and methods can be executed in scouting protocols and queues. With features supporting high operational security, runs can reliably be conducted unattended, for example, overnight.
With ÄKTA avant, developing a method is easy. Processes are quickly created in the UNICORN software by using predefined methods for different chromatography techniques or from phases reflecting individual steps of a chromatography run. Subsequent scaling of your process demands easy technology transfer between scales. For quick process scaling, the software automatically converts and scales methods between ÄKTA avant 25 and ÄKTA avant 150 system versions.
Often when an instrument is shared among many users, getting access to the instrument can be a bottleneck. Installed in a network configuration, methods can be developed, runs monitored, and results evaluated from your office computer. Over the network, developed methods and results are easily shared among users and between instruments.
ÄKTA avant exhibits many features supporting high productivity and efficiency in operations. This white paper describes how ÄKTA avant users take advantage of system features supporting time savings, easy method development, high operational security, and efficient equipment utilization, with the overall objective to gain efficiency in process development.
Fig 1. ÄKTA avant is designed for efficient process development and optimization using the DoE functionality of the UNICORN system control software.
EASY METHOD DEVELOPMENT AT BOEHRINGER-INGELHEIM
Boehringer Ingelheim is a global group of companies committed to researching, developing, manufacturing and marketing novel medications of high therapeutic value for human and veterinary medicine. The businesses comprise prescription medicines, consumer health care, chemicals, and biopharmaceuticals.
Hans-Georg Bischof, Process Development, Protein Science, develops purification processes for biopharmaceuticals at Boehringer-Ingelheim in Biberach, Germany. About 80% of the work comprises purification of monoclonal antibodies (mAbs). The work spans from process development in 10 mL to 80 L scales to pilot-scale manufacturing.
Creating methods
The main task of Hans-Georg Bischof and his team is to develop purification processes for novel products. At manufacturing scale, the overall process needs to be short. About three cell culture batches are harvested each week to gain cost efficiency in the production. Consequently, each downstream purification process, including cleaning and preparation of equipment for the next run, needs to be finalized within 48 hours before the next purification process is started. The process time needs to be considered when developing the purification protocol. For process times to be as short as possible, the runs are performed at the edge of the maximum flow rates of the packed column. To protect the column and resin bed from overpressure under such conditions, the delta pressure control function is used allowing the differential, pre- and post-column pressure to be monitored and controlled.
A large part of the work that Hans-George Bischof and his team perform is on mAbs. As this class of molecules exhibits many shared properties, a platform approach for purification of mAbs is commonly used. Most often, an initial capture step, including a protein A-based chromatography resin, is followed by an anion exchange step in a two-step process. At Boehringer Ingelheim, some processes are complemented with a third hydrophobic interaction or cation exchange chromatography step for virus clearance or DNA removal. The team commonly uses the Method Editor of the UNICORN control software for method development (Fig 2). Predefined methods are used and sometimes subjected to minor adjustments by text programming. Within the mAb platform, methods can often be reused with small modifications, enabling time savings in the process development phase.
“The Method Editor enables quick and easy method development.”Hans-Georg Bischof, Boehringer-Ingelheim, Germany
At Boehringer Ingelheim, many different chromatography resins are screened for optimized performance. However, screening of multiple parameters demands many runs. Consequently, the ÄKTA avant systems are in use day and night at Boehringer Ingelheim. To maximize the amount of information retrieved, while minimizing the number of experiments, the DoE functionally of the UNICORN software is used. Results are exported for visualization and analysis using MODDE™ software (Umetrics AB).
Fig 2 In the Method Editor of the UNICORN software, purification processes are easily created using predefined methods for different chromatography techniques or by dragging and dropping phases reflecting individual steps of a chromatography run.
Sharing of methods and run data
The UNICORN administration module is very flexible and allows many different setups to fit each particular customer. At Boehringer-Ingelheim, the ÄKTA avant systems are shared among a large number of users. To facilitate sharing of methods and data, 22 ÄKTA avant systems are installed in a network accessible to more than 120 users located at different sites in both Germany and Austria. Each site connected to the ÄKTA avant network has its own user group. For each user group, one super user is selected. The super user can, for example, handle installation of new software versions for several systems simultaneously. The administrator makes sure that the systems are regularly backed up by setting suitable intervals for network backups. The administrator also has access to and can share all data among user groups. The solution with super users and one administrator facilitates network maintenance and enables secure storage of all run data.
At the Biberach site, about 50 users are included in the user group. Each user can access all ÄKTA avant systems from their office computer. In a network configuration, methods can be created and runs can be monitored from remote computers.
“Integration of our ÄKTA avant systems in a global network allows for sharing of developed methods among users, supporting global standardization of our processes.”Hans-Georg Bischof, Boehringer-Ingelheim, Germany
HIGH OPERATIONAL SECURITY AT MOLECULAR PARTNERS
Molecular Partners is a company based in Zürich, Switzerland that has established a robust platform to discover and develop DARPin-based medicines. DARPins combine the desired properties of antibody therapeutics with many of the beneficial characteristics of small molecules. Drug candidates targeting indications in ophthalmology, inflammation, and oncology are being developed by Molecular Partners, with the lead product abicipar (formerly MP0112/AGN-150’998) that is planned to enter late-stage clinical trials in two indications: wet age-related macular degeneration (wet AMD) and diabetic macular edema (DME).
Andreas Schweizer is Senior Scientist and Group Leader Downstream Purification at Molecular Partners. He and his team use ÄKTA avant for both process development and preparative chromatography and make use of the many system features for secure operations.
Column traceability
At Molecular Partners the ÄKTA avant systems are shared among several users and columns are reused multiple times. To keep track of column history, Andreas Schweizer and his team use the Column Logbook. The Column Logbook keeps track of every individual column, providing users with column history and performance evolution data. Various maintenance notifications can be set to sustain performance throughout the lifespan of each column.
“The Column Logbook enables tracking of column use, which is important as our ÄKTA avant systems are operated by different users.”Andreas Schweizer, Molecular Partners, Switzerland
Reliable run control
At Molecular Partners, the ability to automate chromatography runs frees up time for other tasks. To reliably run the systems unattended, operational security is supported by system features such as air and pressure sensors. During sample application, the air sensor detects when the sample has been completely injected, even when working with unknown sample volumes, allowing the process to continue to the next step without air being introduced into the flow path. When making use of the large flow capacity of the ÄKTA avant system, the delta pressure control functionality is used to protect the column and resin bed from overpressure. The built-in fraction collector enables automatic detection of deep-well plates, tubes, or bottles and keeps the fractions securely cooled until ready for the next step (Fig 3). As the ÄKTA avant systems are installed in a network configuration, the processes can be conveniently monitored remotely from an office computer
“The network capability is a big leap forward for us, allowing remote access to run data.”Andreas Schweizer, Molecular Partners, Switzerland
Fig 3. The capacious fraction collector keeps fractions securely cooled until taken care of.
Accurate process scaling
Andreas Schweizer and his team use the ÄKTA avant systems not only for screenings of ion exchange resins and method development, but also for scaling up to larger columns. Hence, linear scalability is of high importance. In the UNICORN software, methods are quickly scaled using a time-saving functionality that automatically converts and scales methods between ÄKTA avant 25 and ÄKTA avant 150 system versions.
“For process scaling, the UNICORN control software makes it easy to just send a method to a larger scale.”Andreas Schweizer, Molecular Partners, Switzerland
EFFICIENT EQUIPMENT UTILIZATION AT ROCHE GLYCART
Roche Glycart AG is an antibody engineering power-house within the Roche Group. The company is located in Zürich, Switzerland and has a focus on product development. The company’s mission is to be a leader in developing next-generation engineered antibody products with increased efficacy that address unmet clinical needs.
Erwin van Puijenbroek is Scientist and Team Leader for Research Antibody Production and Purification at Roche Glycart AG. He and his team use ÄKTA avant for screening of protein A-based and ion exchange chromatography resins.
“We usually perform long runs of up to 14 hours and the Method Queues function of the UNICORN software greatly facilitates our work.”Erwin van Puijenbroek, Roche Glycart AG, Swizerland
Unattended operations
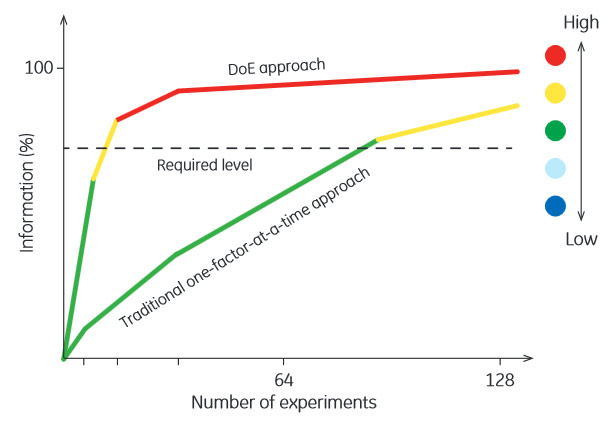
The team uses ÄKTA avant to a large part in DoE studies. Although the required number of experiments to obtain sufficient information about a process can be greatly reduced with DoE, process optimization can still demand a substantial number of runs (Fig 4). To enable unattended operations, a scouting scheme is created to automatically repeat a method with one or more parameters altered between runs. For efficient equipment utilization, runs are often conducted overnight and processes are executed in method queues. With the capacious fraction collector, many fractions can be collected and stored cool until the next morning.
Fig 4. Comparison of the number of experiments required to reach an acceptable level of information in an experimental study.
Remote system access
Erwin van Puijenbroek and his team use ÄKTA avant in a network configuration. Purification tasks can easily be distributed between available instruments and methods can be created and processes monitored from a remote office computer. The network capabilities also facilitate file transfer for data evaluation and analysis. In a network configuration, results are saved locally and securely stored on a database server. For evaluation and analysis of the results, Erwin van Puijenbroek and his coworkers export data for visualization using the JMP™ software (SAS Institute).
CONCLUSION
This article describes how ÄKTA avant system features can be used to gain efficiency in process development and optimization. Chromatography processes are easily created using predefined methods or from phases corresponding to individual process steps. The high degree of automation reduces time for manual interaction with the system, which facilitates the use of ÄKTA avant in applications such as process development. System features such as air and pressure sensors enhance operational security when the system is run unattended. To protect your protein, the built-in fraction collector keeps all fractions securely cooled until ready for the next step. Installed in a network configuration, runs can be conveniently monitored from a remote location and methods are easily shared among users. Processes are quickly scaled using a time-saving functionality that automatically converts and scales methods between ÄKTA avant 25 and ÄKTA avant 150.
With features supporting easy method development, high operational security, and efficient equipment utilization, ÄKTA avant can help you save time and gain efficiency in process development.
We thank Hans-Georg Bischof, Andreas Schweizer, Erwin van Puijenbroek, and their teams for kindly sharing their experience of working with ÄKTA avant. Hans-Georg Bischof, Andreas Schweizer, Erwin van Puijenbroek, and their teams received no financial support from Cytiva for their contributions.