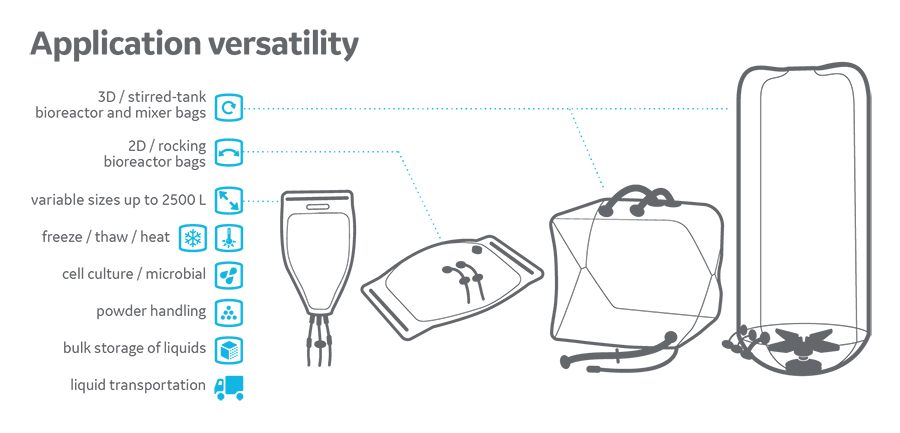
Single-use bioprocess technology offers several advantages for manufacturing biopharmaceuticals, such as a greater diversity of systems to support bioprocessing unit operation (i.e., rocking and stirred tank bioreactor) and enabling transportation of process liquid and drug bulk substance between biomanufacturers’ production sites. Single-use systems (SUSs) are typically fabricated using polymer films containing materials such as polyethylene (PE), polypropylene (PP), or ethyl vinyl acetate (EVA). Composition of the polymer film has a strong influence on the construction, performance characteristics, and manufacturability of the SUS. The majority of polymer films used in SUSs in the market today are adopted from other applications, such as food packaging and blood bags. These films are usually limited to one specific bioprocessing application and are not well characterized in terms of biocompatibility data. Also, these films are typically supplemented with additives to prolong their usability and manufacturability. The presence of additives has resulted in adverse effects of polymer films on cell growth and metabolism.
Reasons to develop a platform film
Cytiva, recognizes the need for a film constructed specifically to meet the complex bioprocessing nature of a multitude of biologics. Hence, Cytiva decided to develop Fortem™ film as a platform film technology across its entire single-use product portfolio. Fortem film is purposefully designed and developed with a carefully selected raw material, incorporating advanced manufacturing technology with an overarching focus on transparency along the entire supply chain.
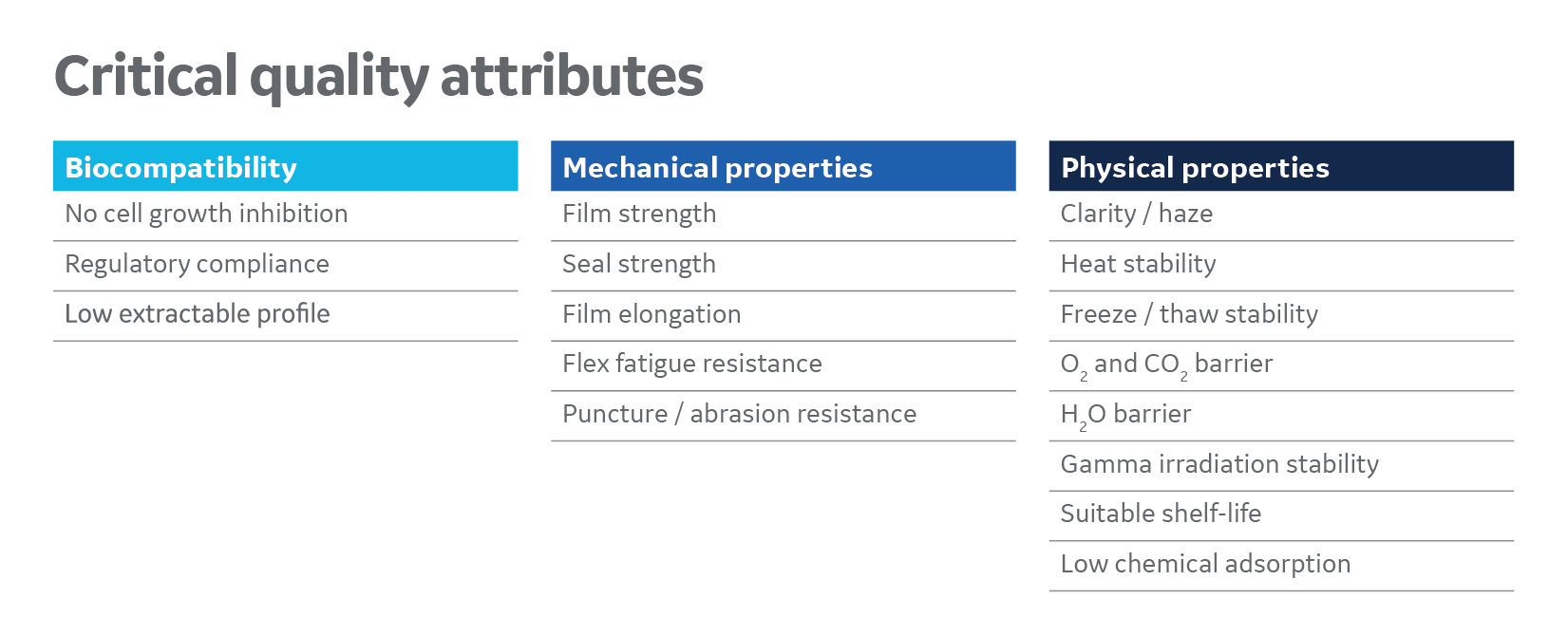
Fig 1. To design a single-use platform film that stretches across bioprocessing, the functional requirements of every application must be balanced against one another, while weighing a host of considerations: choice of polymers, film thickness, flexural properties, degree of crystallinity, resistance to gas transmission, and many more.
Manufacturing technology is one of the critical aspects of producing a quality biologic. When single-use technology (SUT) is deployed in biologics production, biomanufacturers shift part of their supply chain, process technology, and quality control responsibilities to the SUT suppliers. When this happens, a biomanufacturer faces new risks associated with developing its target product within the desired quality and consistency specifications. SUT end users expect their supplier to have a complete understanding of raw materials used for the film, the science behind the film’s composition and characteristics, and the engineering and quality control measures of the film manufacturing process.
The right mindset
Cytiva ensured the following three important strategies were executed to ensure Fortem film was developed successfully: 1) work with a reliable partner with expertise in film manufacturing for pharmaceutical use, 2) actively engage with SUT end users to identify common pain points, and 3) adhere to the development strategy of balancing the film attributes to the material of construction, performance characteristics, and manufacturability of the film and its product.
To acquire this information effectively, Cytiva decided to partner with leading film manufacturer Sealed Air Corporation (SEE), a company well known for its strong material science knowledge and advanced manufacturing technology. SEE shared extensive information about its materials, including information and manufacturing expertise related to each of the layers in the film. One example is the design of the contact layer of Fortem film, which is a blend of polyethylene and cyclic olefin copolymer. The key advantage of this blend is that it does not require small molecule slip agents, as they could potentially become leachables. Furthermore, cyclic olefin copolymers have a benefit of low protein adsorption, making it a suitable material to use for pharmaceutical container technology.
Engineering a bioprocess film
At the beginning of the project, a series of quick pass-fail tests were established to assess whether a film was suitable as a platform film across different application, i.e. cell culture, mixing, fluid storage and transportation and freeze-thaw of formulated bulk solution. Films that were already in Cytiva’s portfolio were assessed and evaluated. Unfortunately, none of those film candidates exceeded the existing stringent criteria for platform film. For example, Bioclear is a good film for WAVE bioreactor application, as it is designed for rocking cell culture operation. However, it is not purpose-built for long-term storage of media or process fluids because it does not have the necessary gas barrier properties required to protect the sensitive chemistries in process fluids over the shelf life. Another example is ReadyKleer film. From a flexibility perspective, it is not ideal to be used in a WAVE platform.
Another top priority was supply chain transparency. As the industry advances toward greater adoption of SUT for GMP production, SUT standards must improve to meet strict cGMP requirements in raw material design, control, quality and continuous process verification. Now, customers have a more significant hurdle with various requirements, and the supply chain has to be developed around those basic requirements. Engineering a film allowed Cytiva to evaluate materials individually before they were constructed into a film prototype. Its construction began at the baseline level of compliance, security of those resins, how they were manufactured, and what information the suppliers are providing to the film manufacturer. That deeper technical knowledge from the start was a critical reason Cytiva decided to create a fit-for-purpose bioprocess film.
Material change challenge
Biomanufacturers typically spend 18 to 26 months and up to $500,000 to qualify a new material that is a direct wetted surface in their process. Typically, three stages are involved in doing this:
- Acceptability of film for general use, assessing material suitability, microbial integrity, extractable study, and toxicological evaluation
- Compatibility with intended process use, assessing chemical compatibility, leachable study, and further toxicological evaluation
- Certificate of assurance verification (multiple lot of SUSs are assessed)
Realizing the customer pain points, Cytiva paid close attention to all the critical film attributes and used test methodologies from recognized industry organizations, such as BPOG, ASTM, ISO, and USP. Customers can utilize as much of this data as necessary to reduce the time needed to qualify the film.
During the development of Fortem film, Cytiva's scientists and engineers designed new test methodologies to gain a deeper understanding of attributes, such as film flexural properties and abrasion resistance, to ensure strong performance under demanding application conditions. Films from multiple lots were tested for consistency and sample sizes were made larger. Cytiva took time with SEE to develop a validation strategy for the film that addressed the various scenarios in which customers would use the film and then created tests that truly correspond to the customers’ processing conditions. The film has been tested in the most rigorous applications, such as integrity at large volume, freezing or high temperature conditions, strength during rocking motion, and bulk liquid transport.
Combined with SEE’s materials proficiency, Cytiva established comprehensive material and film specifications and the film’s performance attributes to address the burden of qualifying a film for general and application specific use.
Determining composition
Approximately 22 film prototypes were evaluated during the research program for Fortem film. Top of mind was compatibility with cell lines pertinent to the biopharma industry. The Cytiva team in Uppsala developed a screening methodology with a very sensitive CHO cell line that allowed them to look at the impact of any potential extractables from these prototypes on cell growth performance and viability. That was an important first consideration that helped to evaluate that aspect relatively quickly.
In addition, some of the more physically rigorous applications of SUT involved a lot of flexing. For example, bags are in motion in WAVE bioreactors. Also, there are large bags for shipping HyClone media and other fluids, which can create significant force during transportation, requiring flexibility in the film. With Cytiva Research Center, a flexibility model was developed that would rigorously flex the material, so prototype performance could be compared against benchmarks known to do well in those applications.
Other areas evaluated were the ability to make strong perimeter seals and port seals with the film, using accessory parts (ports and manufacturing equipment) to ensure the bags could remain integral under various strenuous conditions. In addition, creating bags from the film was considered, as well as burst testing and other types of testing such as pressurization, freeze/thaw cycling, and adding physical and mechanical forces that films would typically experience. In some cases, conditions were exaggerated to ensure requirements were not just being met for various applications but were exceeded.
Transport of fluid
Besides cell culture, film properties were considered that are important for storing and shipping media, for buffer and process liquid applications, and, in particular, for gas barrier properties. The film must be able to support a shelf life of one, two, or three years, depending on the product, making stability another important factor to consider.
One aspect of stability relates to exchanging gases between the environment and the product within the bag. It can be detrimental to have too much oxygen permeate the material because this could lead to degradation of the more sensitive chemistries in cell culture media. In addition, CO2 exchange is an important consideration due to the fact that several products have a carbonate buffer. CO2 exchange becomes potentially problematic from a pH stability perspective. Furthermore, when considering accumulative shelf life over time, there should be a concern over loss of volume related to exchange of moisture vapor. Some challenges were faced during development. For instance, the first version film candidate was not necessarily the best option for process liquid storage and shipping due to its gas barrier properties. An earlier design of experiment approach ensured that the film formulation was optimized within a short period of time.
Additional tests on the road to finalization
One of the challenges of testing small-scale bag assemblies is that they do not always translate to large-scale. Various tests were completed with 2,000-liter (L) bioreactor bags, including extensive pressure testing, which is an exaggerated test to ensure the film would be able to withstand the forces of the liquid for the extended period of the cell culture test. Temperature testing was performed from freezing down to minus 80 degrees Celsius, as well as a simulated application in a 500 L fermenter with temperatures from 37 to 60 degrees Celsius.
Additional testing was completed to observe Fortem film’s behavior under a high rate of agitation at higher temperatures over a long duration. The goal was to see how the film behaved, if the integrity would be maintained, and if there would be any issues with it being able to carry out the run for an extended period of time. Situations in which the bags were unsupported were also examined to ensure the integrity of the systems could be maintained in those conditions.
Achieving flexibility while optimizing film characteristics can also be challenging. Sometimes that means certain characteristics are sacrificed, such as stiffness. As the stiffness characteristics are reduced, there is potential to have “creep” in the film, which is the material pulling downward and getting longer. By testing these factors in exaggerated conditions, it ensures that Fortem film can withstand the normal conditions in which customers use it.
Conclusion
Cytiva is committed to its single-use solutions and has been relentless over the years in pursuing a strategy to enhance quality, supply assurance, and change control for its single-use bioprocessing products. Fortem film development is an important investment by Cytiva, as it helps meet the industry need for a fully characterized platform film across all bioprocessing applications with supply chain transparency as a focal point.