Reduce risk of failure in your virus clearance study by choosing robust scale-down chromatography tools
Tina Pitarresi and Linnea Troeng
Cytiva Bio-Sciences AB, Björkgatan 30, 751 84 Uppsala, Sweden
Introduction
Effective viral clearance studies remain one of the challenges facing manufacturers of biologics. These studies are an essential part of process validation and are critical to ensure drug safety. Viral clearance studies are usually performed using a scale-down model mimicking the large-scale process step. For chromatography steps, using a scale-down model limits the amount of sample and resin used, lowering the cost for the study. As viral clearance studies are often cost and time consuming, it is essential to minimize risk of failure.
Seven considerations for performing viral clearance studies on chromatography steps
There is a lot to keep in mind before designing your study. Here are seven things to consider for your viral clearance study column:
- Select a suitable scale-down model
- Use a column with a narrow inner diameter (Fig 1)
- Know when to choose prepacked or empty columns
- Do not compare compression factors
- Challenge your chromatography column
- Perform an integrity test on the column
- Use manufacturing feedstock
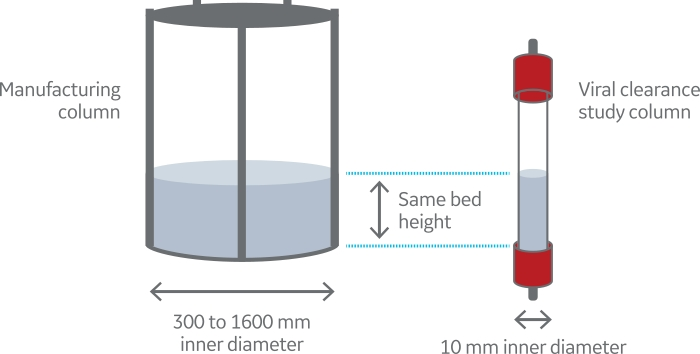
Fig 1. Schematic description of scale-down chromatography mode
High-quality column packing reduces risk of failure for robust viral clearance studies
Several chromatography columns are required for each viral clearance study. So, it is essential to carefully consider which columns to use.
Remember that the column should:
- be easy to pack or be prepacked
- provide reproducible results between runs
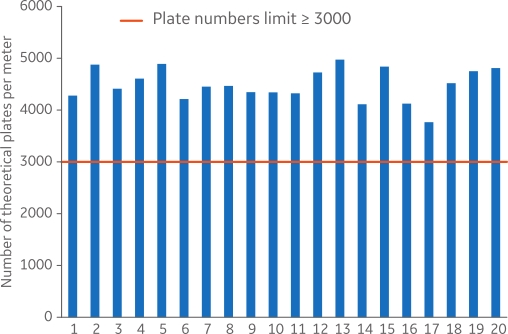
Figure 2 shows efficiency analysis for 20 different MabSelect SuRe Validation columns featuring high-quality packing with theoretical plate numbers per meter above the limit (3000) and consistency between the packed columns.
Fig 2. Efficiency analysis of 20 MabSelect SuRe Validation column
Case study: a successful viral clearance study for a protein A chromatography step
In this study, the virus reduction of an affinity capture step was evaluated using HiScale 10/40 columns packed with MabSelect PrismA resin. The study was performed by a third-party viral testing laboratory in collaboration with a biopharmaceutical company. The model viruses used were Xenotropic murine leukemia virus (xMuLV) and Minute virus of mice (MVM).
Design of experiments to challenge viral clearance robustness
To challenge the method robustness, different scenarios were studied where the sample load and residence time (RT) varied (Table 1). The standard set up was run in duplicate. The resin bed height and column volume were approximately 20 cm and 16 mL, respectively.
Results and discussion
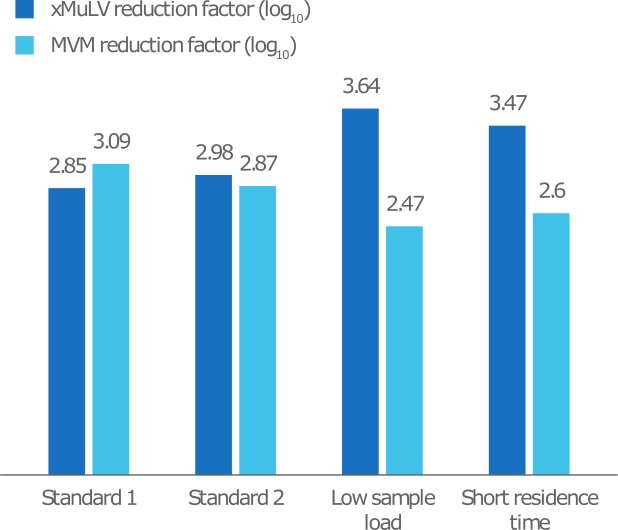
Overall, the reduction factors observed in this study were within the typical range found for protein A resins (typical log reduction factor in the range of around 2.5). This demonstrates expected virus removal and a robust process even when sample load and residence time are varied.
This study shows that HiScale 10/40, packed with MabSelect PrismA, is a reliable choice for the capture step in a virus clearance study. Together, the column and resin offer good viral log reduction in a suitable scale-down column format
Fig 3. Summary of reduction factor results in relation to sample load and
Table 1. Details of the different scenarios studied
| Scenario | Sample load concentration (mg/mL) | RT in chromatography process (min) | RT in loading step (min) |
|---|---|---|---|
| Standard 1 | 65 | 6 | 12 |
| Standard 2 | 65 | 6 | 12 |
| Low sample load | 20 | 6 | 12 |
| Short residence time | 65 | 4 | 4 |
View the full case study: virus reduction of an affinity capture step
When to use prepacked columns or pack your own?
| Add flexibility with empty HiScale 10/40 columns | Save time with prepacked Validation columns |
|
|
Summary
- Scale-down columns are commonly used for resin viral clearance studies.
- Both the empty HiScale 10/40 columns and the prepacked Validation columns are well-suited for scale-down studies.