Large-volume cryopreservation
Cryopreservation of small sample volumes is common in many scientific applications. However, there is increasing demand for large-volume cryopreservation, especially in cell and gene therapies, where treatments require large cell numbers.
Although cryobags are generally a good choice for the cryopreservation of large-volumes, cryovials provide a more robust alternative. They are also considerably easier to fill and empty, and are available in volumes up to 50 mL to meet high volume needs. However, the thermal performance of large-volume cryovials differs markedly from small vials and cryobags, which have wall thicknesses of typically 100 to 300 µm and so enable rapid heat transfer in and out of a biological sample.
By comparison, large-volume vials require thicker walls, typically up to 1 mm, to maintain structural security during cooling, thawing, storage, and transportation, and are made from polymers with poor thermal conductivity. This combination results in delayed heat transfer in and out of a sample, which can lead to thermal gradients within a large-volume sample.
The resulting cooling inconsistencies mean cells in the center of a vial can freeze more slowly than those nearer the edge, affecting their viability and function (1).
Such inconsistent cooling is also a consideration when freezing cells with liquid nitrogen (LN2), often the default choice for cryopreservation. Samples nearest to where the liquid nitrogen is pumped into the cooling chamber cool at a different rate to samples further away. As well as potential sample variability, liquid nitrogen gives rise to concerns about contamination and sterility, safety, and high running costs.
This study demonstrates an optimized cooling protocol for cryopreserving large-volume cryovials in a VIA Freeze Quad controlled-rate freezer, avoiding the need for liquid nitrogen. Using a new sample plate, developed to accommodate large cryovials and increase the capacity of the VIA Freeze Quad freezer to 16 vials and a total of 480 mL, this protocol produces consistent cooling profiles. Samples are cooled evenly below the -60°C necessary for cryopreservation and show rapid recovery and post-thaw viability.
Methods for cell freezing protocols and analyses
Investigating thermal variation
Table 1 outlines an optimized cooling protocol for large-volume vials, refined to account for vial wall thickness, thermal conductivity of vial sample plates, and known biological constraints.
An initial temperature of -2°C is optimal for sample loading, as this is too warm for ice to form, but could prevent excessive DMSO toxicity.
The plastic walls of the vials result in a thermal lag between freezer and sample temperatures. This is expected to be less than 10°C during the cooling stages and is accommodated by a 10-minute hold so as not to substantially affect overall cooling rates.
The hold at -30°C allows for any thermal deviation during ice nucleation to settle. Finally, the 10-minute hold at the end of the second cooling step makes sure all samples are below -60°C. As the intra-cellular glass transition is known to occur above -50°C, -60°C represents an acceptable temperature for transfer into long-term storage.
Table 1. Cooling protocol optimized for large-volume vial-based cryopreservation
| Cryopreservation step | Purpose |
| Room temperature to -2°C at 1°C/min | The initial cooling to -2°C to minimize DMSO toxicity |
| 10-minute hold at -2°C | A hold at -2°C to accomodate the thermal lag from thicker plastic cryovial, resulting in a liquid temperature just above 0°C, and allow sample loading |
| -2°C to -30°C at 1°C/min | Freezing of samples, reducing temperature to below their equilibrium freezing point where ice starts to form |
| 30-minute hold at -30°C | A hold to allow the whole sample to freeze after ice nucleation |
| -30°C to -70°C at 0.75°C/min | Cooling resumes when samples are macroscopically frozen until temperature falls linearly to -70°C |
| 10-minute hold at -70°C | A final hold enabling the vials to equilibrate before safe transfer to LN2 at this temperature |
Two thermal tests demonstrated how this optimized cooling protocol accounts for the potential effect of thermal variation using large-volume cryovials.
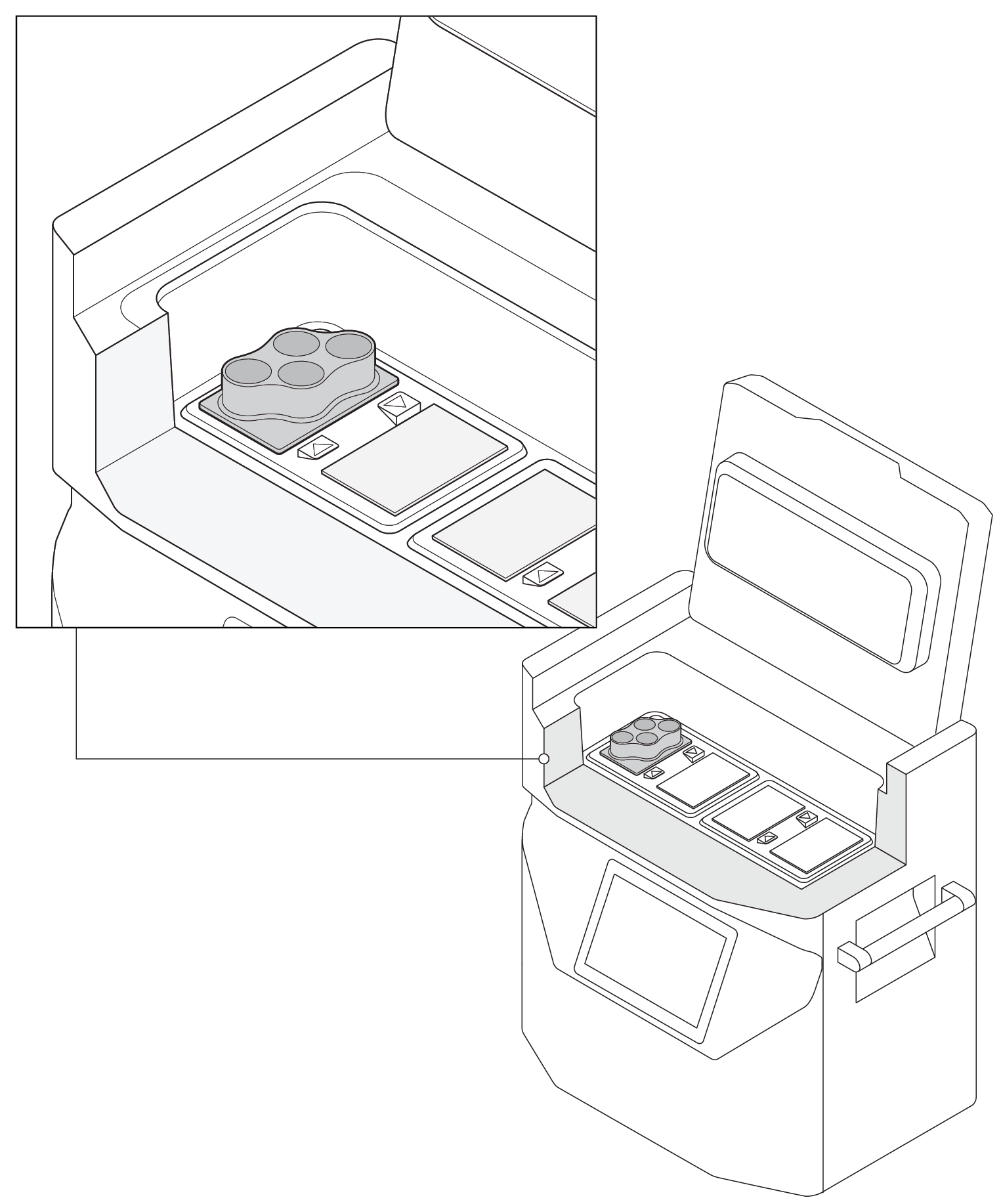
Sixteen 50 mL AT-Closed Vial cryovials (Aseptic Technologies) containing 30 mL culture medium and 5% (v/v) DMSO were placed into four multi-vial sample plates (Cytiva) (Fig 1) and positioned in a VIA Freeze Quad controlled-rate freezer (Cytiva).
For the first thermal test, t-type thermocouples were randomly placed within the medium of eight vials to measure any inter-sample variability and the impact of thermal lag through the cryovial walls.
In the second thermal test, thermocouples were placed in two of 16 new cryovials in the VIA Freeze Quad freezer to measure intra-sample variability when cooling large 30 mL volumes. One thermocouple was placed near the base of each vial, two towards the middle, and a fourth near the upper surface of the liquid.
Freeze cells in 50 mL cryovials without liquid nitrogen using this controlled-rate freezer to achieve high post-thaw recovery of biological samples.
Fig 1. A cutaway image of the VIA Freeze Quad controlled-rate freezer loaded with one multi-vial sample plate (up to four of these sample plates can be accommodated). The VIA Freeze Duo freezer can accommodate two sample plates (eight vials) and the VIA Freeze freezer has capacity for one sample plate (four vials).
Evaluating post-thaw viability
To evaluate post-thaw viability with the refined cooling protocol, four vials were filled with 30 mL suspensions of Jurkat cells at a density of 107 cells/mL in RPMI-1640 (Thermo Fisher Scientific), containing 10% HyClone Calf Serum (Cytiva) and 5% DMSO, as well as 12 vials containing only medium and DMSO. These were placed and cooled in the VIA Freeze Quad freezer as per the protocol in Table 1 before being transferred to ultra-low temperature storage at -140°C.
After a minimum of 24 h storage, samples were thawed in a 37°C water bath for approximately 16 min and re-cultured.
Viable cell count through fluorescein diacetate (FDA) staining was assessed at 24, 48, and 72 h post-thaw using a Cytell Cell Imaging System (Cytiva). Cell functionality was also determined with a 4 h 30 min Alamar Blue relative fluorescence assay using a GENios FL plate reader (Tecan), which provides a more in-depth analysis compared with viable cell number alone.
The samples were compared to an unfrozen control at all time points and experiments performed in triplicate.
Results for cryogenic processing
Thermal variation during cryopreservation
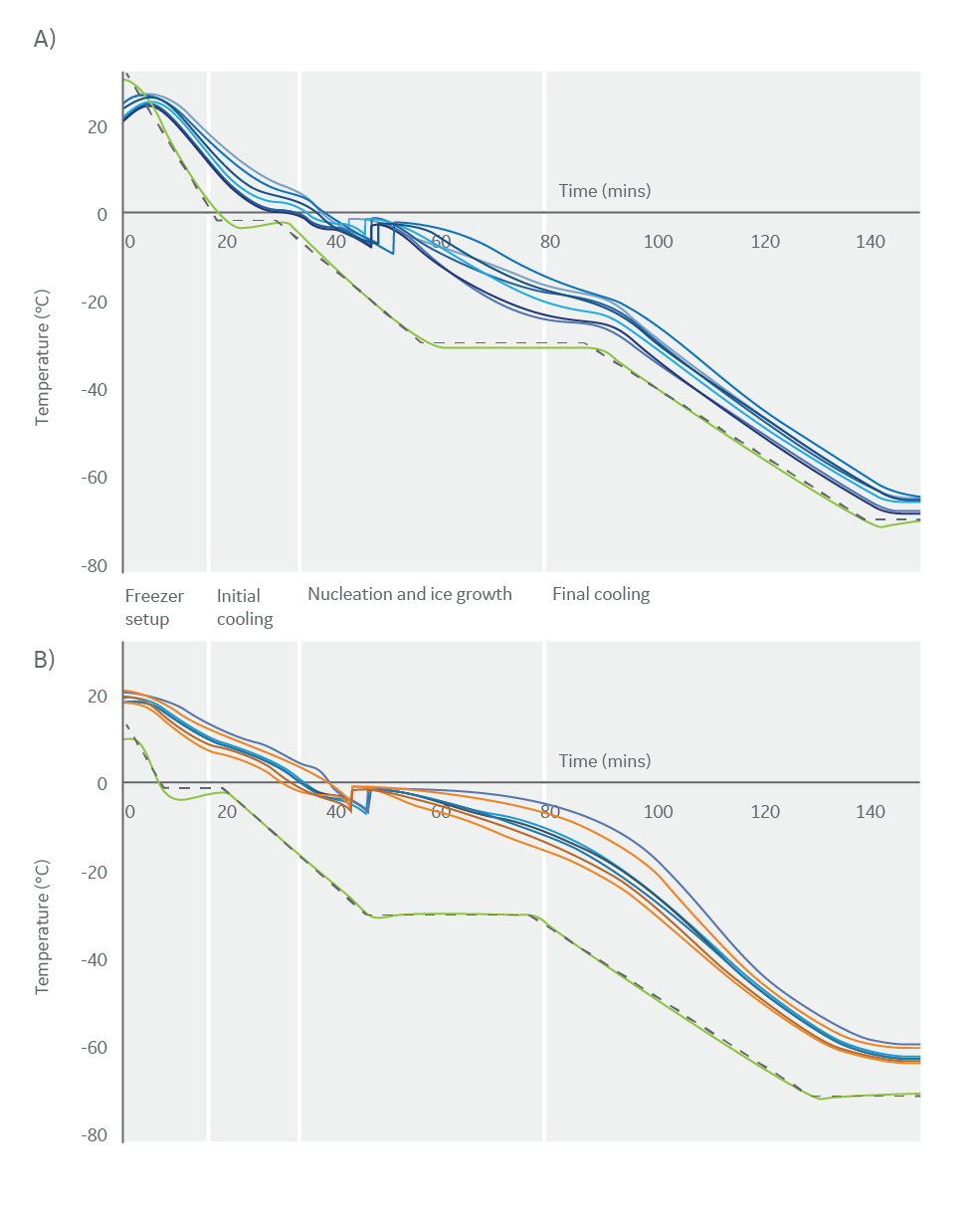
The optimized cooling protocol helped maintain consistency between samples, as demonstrated by minimal inter-sample differences during cooling events in the first thermal test (Fig 2A). Some deviation did occur during nucleation; however, this is expected in all types of vial due to the stochastic nature of ice nucleation (2), and allowed for through the hold step at -30°C.
There was also minimal intra-sample thermal variation in the second thermal test (Fig 2B). The variations seen within the two vials containing thermocouples is comparable to the intra-sample differences. These results suggest that the differences are a consequence of well-known intra-sample variation (1), as thermocouples are difficult to place in the exact same location in different samples.
Fig 2. The thermal profiles observed during a 16-vial cryopreservation run, showing programmed cooling profiles (black) and freezer temperatures (green). (A) Thermal profiles observed for eight large-volume vials (blue) to evaluate inter-sample variability during cryopreservation. (B) Thermal profiles observed for two large-volume vials (vial one in blue, vial two in orange), each containing four thermocouples to evaluate intra-sample variability during cryopreservation.
Biological post-thaw viability following cryopreservation
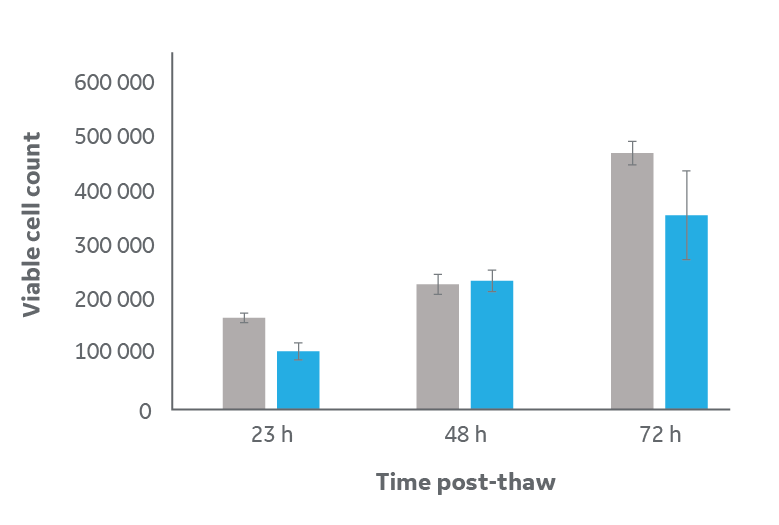
The number of viable cells recovered after 24 h of culture post-thaw was 74% ± 16% compared to the unfrozen control (Fig 3A). This viable cell count is consistent with the expected impact of cryopreservation and culture interruption.
The subsequent strong growth in cell number is observed across all samples, with fold changes of 1.9 ± 0.3 and 3.2 ± 0.9 after 48 and 72 h of culture post-thaw respectively, compared to the 24 h time point. These results indicate that cryopreservation in vials using the optimized protocol does not inhibit proliferation and results in no noticeable delayed onset cell death.
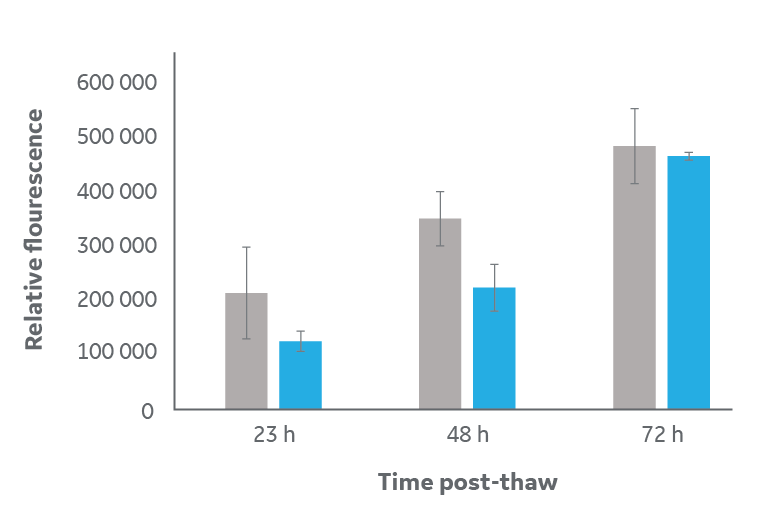
Post-thaw, cryopreserved cells have ≤ 24 h delay on matching the metabolic activity of the unfrozen control, followed by a rapid recovery after ≥ 48 h (Fig 3B). This recovery period is also consistent with the expected impact of damage caused by cryopreservation and culture interruption. The Alamar Blue data also supports the conclusion that, post-thaw, cryopreserved cells are healthy as they continue to metabolize and proliferate.
Fig 3. Analysis of cell viability and metabolism for samples frozen in large-volume cryovials (blue) compared to unfrozen control (gray). (A) viable cell count via FDA staining, and (B) metabolic activity read by Alamar Blue relative fluorescence activity. Cells were thawed then cultured under normal conditions for 24, 48, and 72 h. Data is n=3 technical replicates ± one standard deviation of mean averages.
Conclusions on cell freezing in large-volume cryovials
Despite the thickness of large-volume cryovials, the thermal tests demonstrate that effective cooling can be achieved with an LN2-free VIA Freeze Quad controlled-rate freezer with a few simple adjustments to a standard cryopreservation protocol, such as lowering the start temperature and allowing for a thermal lag at key stages.
The optimized protocol outlined in Table 1 enables consistent cooling profiles and, while a thermal lag is apparent, tests indicate that there are no major inter- or intra-sample thermal variations. The samples can safely cool to below -60°C, a temperature suitable for biological cryopreservation, without incurring unexpected damage. Similar cooling profiles are known to be acceptable for T cell cryopreservation (3).
The biological test data demonstrates that interrupting the culture and cryopreserving samples using this protocol does not affect cell viability and metabolism. Cells recover from any delay in proliferation compared to unfrozen controls within 24 h post-thaw.
References
- Kilbride, P. et al. Spatial considerations during cryopreservation of a large-volume sample. Cryobiology 73, 47–54 (2016).
- Morris, J.G. and Acton, E. Controlled ice nucleation in cryopreservation–a review. Cryobiology 66, 85–92 (2013).
- Baboo, J. et al. The impact of varying cooling and thawing rates on the quality of cryopreserved human peripheral blood T cells. Sci. Rep. 9, 3417 (2019).
Read about large-volume multi-bag cryopreservation.
Explore Cytiva solutions for cryogenic cold chain management.
Ordering information
| Product | Product code |
| HyClone Calf Serum, U.S. origin | SH30073.03 |
| VIA Freeze Quad Controlled-Rate Freezer | VFQ_30010 |
| VIA Freeze multi-vial sample plate | Estimated available Q3 2019* |
*Coming soon. There is no guarantee regarding the release of any products (Cytiva reserves the right to change plans and timing in regards to the release of any products).