Alternative to water baths for thawing frozen cells
How cells are thawed determines their viability and function. Conventionally, cells are thawed in a water bath at 37°C. This practice is problematic, because water baths pose contamination risks. They also require calibration and validation. Most importantly, water baths do not provide a record of the thaw process.
Asymptote has developed dry thawing systems that overcome many of the issues inherent in water baths. These thawing systems also enable accurate data logging. VIA Thaw CB1000 is designed specifically to thaw cells in bags (cryobags).
This benchmarking study compares performance of the VIA Thaw CB1000 system and a water bath for thawing frozen cells. The samples were mobilized apheresis samples from patients with multiple myeloma or non-Hodgkin’s lymphoma. CD34+ and CD45+ cell recoveries were determined after thawing. These cell types are widely used as markers for the quality of mobilized apheresis samples.
Fig 1. VIA Thaw CB1000 automated dry thawer.
Benchmarking study details
Apheresis samples
Apheresis samples were collected from nine patients in remission from multiple myeloma or non-Hodgkin’s lymphoma.
Cryopreservation
A 20% dimethylsulfoxide (DMSO) solution in 4.5% human albumin was used as cryoprotectant. The solution was added slowly to each sample at a 1:1 ratio to make a final volume of approximately 40 mL per cryobag in 10% DMSO. Up to eight cryobags (Baxter Cryocyte or OriGen CryoStor™, overwrapped in a thin nylon/polypropylene plastic mixture) per patient were cryopreserved.
Samples were frozen in a controlled-rate freezer (Kryo 10, Planer PLC) according to the following temperature profile:
- Hold at 4°C for 10 min
- Cooling rate of 2°C/min to -30°C
- Cooling rate of 4°C/min to -100°C
- Hold at -100°C until transfer to the vapor phase of a liquid nitrogen storage tank
Storage, transportation, and thawing
Samples were stored for up to 17 years.
A pair of cryobags from each patient was removed from storage. The bags were transported to the laboratory in a dry shipper with temperature monitoring.
Following laboratory standard operating procedures, one bag from each pair was thawed in the CB1000 thawer and the other in a water bath.
Analysis
After thawing frozen cells, total viability was assessed by trypan blue exclusion. Component viability (CD34+ and CD45+) and cells counts were determined by flow cytometry. Paired t-tests were carried out on the data, with p < 0.05 used to indicate significance.
Results
Results are presented as mean ± standard error of the mean (SEM). No statistically significant differences were observed between the two cell thawing conditions for total viability (trypan blue) or CD34+ and CD45+ component viability (Fig 2).
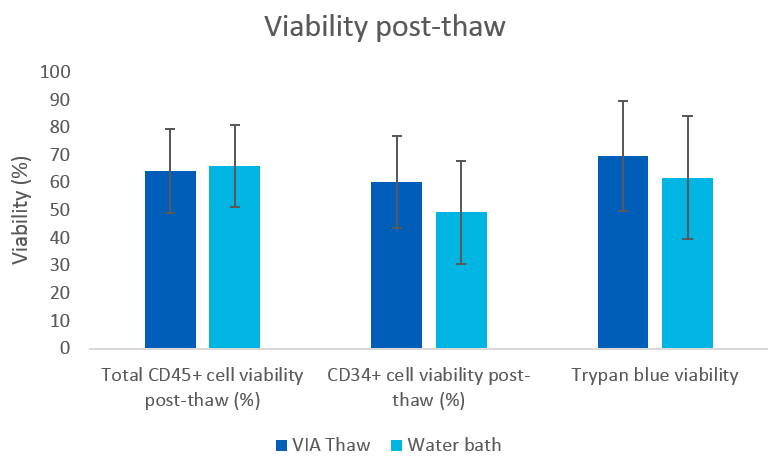
Fig 2. Peripheral blood mononuclear cell (PBMC) viability measures after thawing in a water bath or VIA Thaw CB1000 system (mean ± SEM), n = 9.
Differences in total CD34+ and total viable CD34+ cell counts were not statistically significant, as shown in Figure 3.
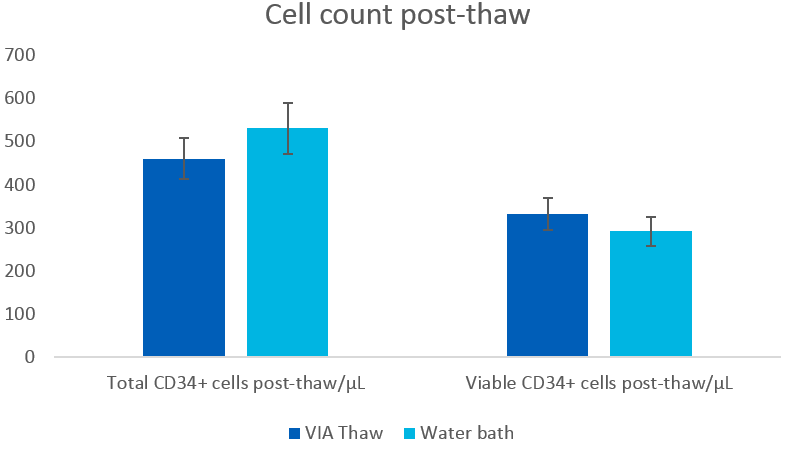
Fig 3. Cell concentration after thawing in a water bath or VIA Thaw CB1000 system (mean ± SEM), n = 9. Cell counts performed by flow cytometry.
Conclusion
This study demonstrated similar cell viability and density after thawing cryobags using either method. Therefore, the VIA Thaw system provides equivalent performance to the water bath benchmark, while improving convenience, consistency, and visibility. The dry thawing technology eliminates water-borne contamination risks and streamlines cell thawing. The automated system provides consistent results that are not operator dependent and logs the thawing process.
Learn about GMP-ready systems for freezing and thawing of cryopreserved cells.