Background on thawing
Temperature-sensitive cell therapies are transforming clinical care, making continuous cold chain management critical in the delivery of treatments to patients. From apheresis collection to clinic, control of cryobiological storage conditions is complicated due to the multistep processes involved in creating a finished cell therapy combined with inevitable uncertainties around the time and place of administration. Cryopreserving therapies below -120°C has been found to offer stable, extended storage in a cell bank until treatment is required.1–3
Cold chain management includes three key phases:
- Cooling
- Storage*
- Thawing1,3–5
For clinical cell systems, the first two phases are usually precisely controlled and recorded using validated protocols and automated controlled-rate freezers.1,6 Automatic alarms and monitoring systems are also essential for ensuring stable storage conditions in cell banks.1,7
Efficient thawing, with minimal impact on viability and performance, is a process gatekeeper and often the final manipulation carried out at the point of care. Errors in thawing of a potentially lifesaving therapy could compromise treatment efficacy, leading to significant patient impact as well as high costs and a compromised reputation for the product’s developer.
For gene-modified immunotherapies and cell therapies requiring highly specialized manufacturing, the starting biological material might be cryopreserved immediately post-acquisition and then shipped to the processing site. The sample is then thawed and processed (e.g., isolation of the cell population of interest, expansion in a bioreactor, activation or gene transfer/editing, and harvesting) before being cryopreserved again for a second cryo-shipment back to the patient site prior to the final thaw and administration.1,5,8,9 Both cryopreservation and thawing occur at multiple steps in the workflow (Fig 1), and any deleterious effects resulting from these processes should be minimized wherever possible.
This guide summarizes the science of thawing following conventional, slow freezing methods. We address how cell thawing has historically developed into the new techniques used today, along with the physical and biological implications of key metrics and components, such as warming rate and ice structure. Also included are reviews of key studies from scientific literature, and a consideration of the interactions between cooling and warming rates, as applicable to cell and gene therapies. We do not consider ice-free cryopreservation (sometimes referred to as “vitrification”), a specialized method unsuitable for most cell therapies and cryobags.
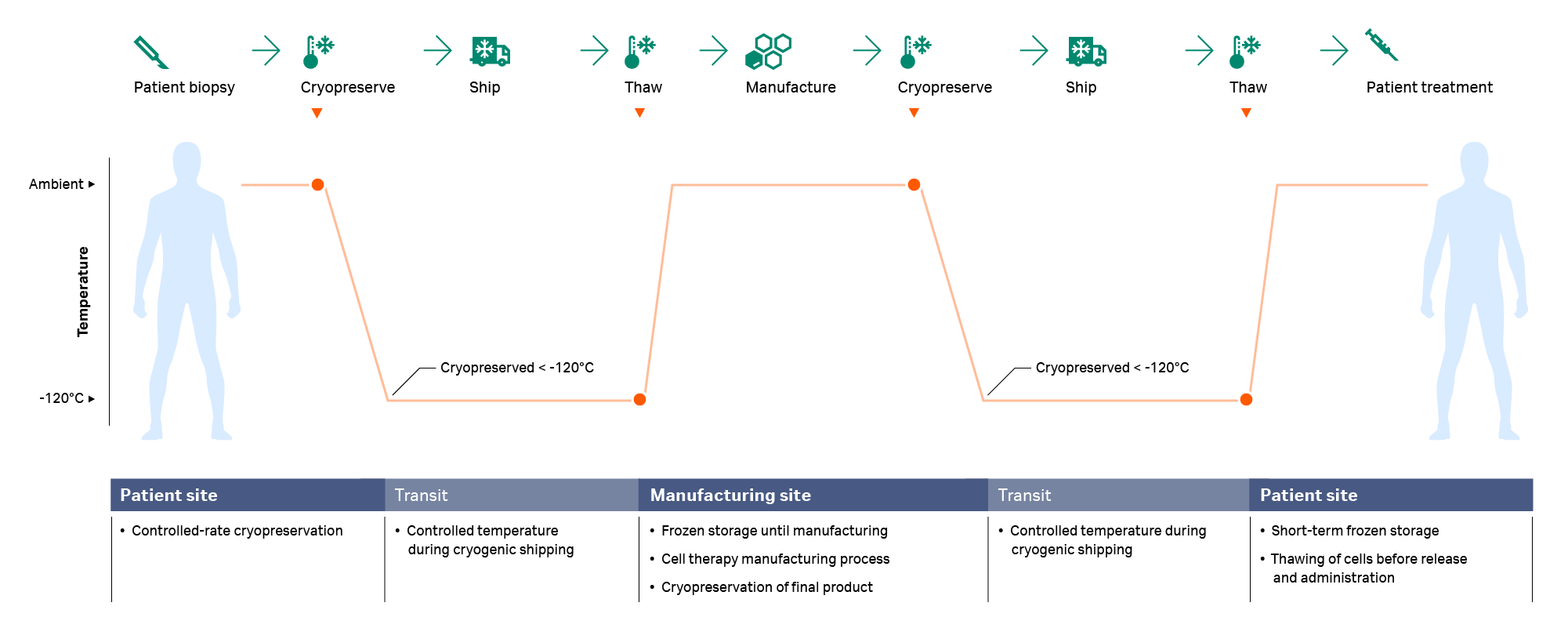
Fig 1. A typical cryochain — the thawing step usually happens twice during the cell therapy manufacturing process: after arrival of the starting material at the manufacturing site and prior to infusion.
History of thawing — why we do it the way we do
Thawing processes for cryopreserved materials have historically involved a relatively simple procedure with a strong, subjective element. Typically, a frozen sample is submerged in a water bath at 37°C with a visual determination of when the last ice has melted (wet thawing).6,10 This method is simple, cheap, and established, but requires a specialized technician to yield success and consistency. Variability in user behavior and laboratory settings can cause batch-to-batch variation in product temperature management. Untrained operators might choose slightly different, and often subjective, cryobag angles, agitation speeds, and end-of-thaw criteria, producing a spectrum of inconsistent results.
Specialized technicians are not always available for thawing at clinical sites, particularly when risk reduction efforts lead to a therapy being delivered in multiple doses (more than one cryobag). Consequently, thawing at patient sites is increasingly carried out by clinical staff who may have minimal training and experience in cryopreservation11, increasing the risk of mishandling, reduced cell viability, and compromised therapeutic performance.
While sufficient in a research setting, water baths present inherent difficulties in clinical and cleanroom environments. In addition to the risk of process and product variability, the presence of warm water introduces a contamination risk that is unacceptable in controlled settings.12–14 Time and facilities for sterilization, rewarming, refilling, and temperature stabilization must also be available.
In response to these limitations, water-free thawing devices capable of handling larger samples held in cryobags are gaining favor.1,5,15–18 These systems eliminate user-to-user variability and provide a consistent, programmable process that minimizes subjective user interventions, including options for computerized control, monitoring, and data recording. Studies have indicated that dry thawing can also be successfully applied to non-cellular therapeutic materials such as plasma1,15–18, further motivating the widescale adoption of these devices.
The science of thawing rates
Controlled cooling for stable thawing: Cooling and thawing rates are intrinsically interlinked, and consideration of one cannot take place without the other. Historically, cryobiologists have thought that rapid thawing was essential for optimal cell recovery.19–21 This dogma has been overturned with more recent developments and is only applicable to very rapid cooling rates not acceptable for cell therapies.1,4 Many myths regarding warming still exist, possibly for the following reasons:
- Lack of active research: The last systematic study on mammalian somatic cells was published over 30 years ago in 1979.22 By contrast, over the same period, at least six papers were published on warming rates for cryopreserved sperm and at least five were published on warming rates for cryopreserved embryos.
- Misapplication of available data: There has been an assumption that data from sperm and embryos translates to somatic cells.
- Delayed washing of cells post-thaw: DMSO toxicity can damage cells post-thawing before the DMSO is washed out. Some procedures considered the physical warming and washing of the cells as one “thawing” step. Delayed washing was sometimes confused with slow thawing.
Wet thawing over the course of approximately 2 to 3 minutes has worked conveniently in the laboratory; however, this rapid approach is not required when thawing somatic cells that have been cooled at a precisely controlled rate. Slower warming rates following slow cooling have been tested for a range of cell types, showing no impact on the post-thaw outcomes for hepatocytes23, neural cells24, T cells4, Chinese hamster ovary (CHO) cells25, L cells22, unprocessed apheresis5, and lymphocytes26. Surprisingly, no study has shown that rapid thawing is required for cell therapy processes/immunotherapies despite the commonly held belief that rapid warming is favorable.
It is frequently assumed that rapid thawing is required to prevent ice re-crystallization on warming. The above cited works have shown that with properly controlled cooling, ice re-crystallization is not possible, regardless of warming rate.
Ice formation during cooling: During cooling, ice forms on nucleation and develops through a system — initially as small dendritic crystals and physically appearing as thick interlinking and variously orientated needles. As cooling progresses, water molecules adhere to the ice crystals and the individual crystals grow in size. This process results in an increased extracellular osmotic concentration, which drives the dehydration of cells. If cooling progresses slowly at a rate of a few °C per minute or lower, crystals have sufficient time to fully form and cannot recrystalize at thawing.
Problems can arise when cooling proceeds too quickly, including damage to cells.4 With rapid cooling, water molecules do not have enough time to adhere to ice crystals or flow out of cells. When temperatures reach the glass transition (around -120°C) and no more molecular movement is possible, crystals can exist in a suspended state with an unstable structure.3 Then once the system warms and heat is re-introduced on thawing, water molecules start to adhere to the ice crystals again.28 This phenomenon, known as re-crystallization, puts substantial osmotic stress on the cells and results in damage.
Fortunately, the slow cooling rates required for cryopreserving cell therapies and somatic mammalian cells (up to a few degrees per minute) allow for the formation of thermodynamically stable ice crystals and negate the need for rapid warming.4 This process can be observed through cryo-microscopy on ice crystals while cooling and thawing at different rates (Fig 2) and has been found to correlate with cell recovery outcomes.
A recent cryo-microscopy study revealed that loss of viability was associated with changes in the ice crystal structure during warming.4 At high cooling rates (-10°C /min), the ice structure appeared highly amorphous. When subsequently thawed over a longer timeframe (15 minutes or more), ice recrystallization was observed, suggesting mechanical disruption of the frozen cells. Cooling at a more typical -1°C/min resulted in no change in the ice structure when warming slowly. By comparison, warming from LN2 temperatures in two minutes in a water bath led to a warming rate of about 100°C/min. More steady warming taking 10 minutes (a typical process duration for dry thawing devices) has an average warming rate of 20°C/min. Both approaches are much faster than a controlled cooling rate and are considered rapid compared to what is physically and biologically recommended.4
Dry thawing of cryobag samples of different volumes cooled around -1°C/min can take longer than thawing in a conventional water bath but results in equivalent post-thaw cell yield and removes the risk of inconsistencies associated with wet thawing in a water bath.

Fig 2. Cryo-microscopy images taken during the cryopreservation process, showing how ice changes occur during cooling and warming. Samples labeled blue were cryopreserved very rapidly, so ice was not fully formed when at the storage temperature (blue image #2). The ice structure changes – sometimes called recrystallization – in thaw, just after blue image #5. Conversely, cells cooled in a controlled manner (orange images #1–#4) have enough time to form large ice crystals on cooling, so no recrystallization is observed upon warming (orange image #5).4 Ice recrystallization occurs during warming, not cooling — the orange figures show how ice can be optimally controlled on cooling to prevent change on warming.
Post-thaw toxicity: In addition to avoiding ice structure changes, it is important to minimize cryoprotectant toxicity. During cryopreservation, cryoprotectant chemicals are added to protect cells from damage, with DMSO being the most used substance for this purpose. In the liquid state, these chemicals are toxic to cells29,30 and that toxicity becomes more pronounced at higher temperatures. Toxicity can be minimized by adding a cryoprotectant at cold temperatures, a few degrees above freezing point, immediately before the freezing process30–32; however, toxic effects resume with thawing and increasing temperatures.
Post-thaw cryoprotectant toxicity has the same damaging potential at all liquid phases of the cryopreservation cycle but is commonly overlooked. Cells are at least as sensitive to cryoprotectant toxicity on thawing as they are on cooling, if not more, due to the fact that they are already stressed by the freeze/thaw cycle.30–32 Some buffer time is still acceptable but varies by product. For example, the manufacturers of commercially available Kymriah™ and Yescarta™ CAR-T therapies recommend infusing the cells into the patient no longer than 30 minutes and 3 hours, respectively, after being thawed in their cryoprotectant medium at room temperature (20°C–25°C). 33–34 This time limit includes any interruptions during infusion and helps ensure maximum viability is maintained.
Thawing for too long in a water bath can adversely impact cellular recovery, as samples will be warmed to 37°C very rapidly due to the high thermal conductivity of water. This rapid warming process can generate large thermal gradients within the bag — small ice crystals can remain in some areas, especially around connectors, while other areas have already reached the water bath’s temperature. Mitigation strategies for wet thawing include agitating the bag during warming and removing it from the water bath just before all the ice has melted. Such measures are, however, user-specific and prone to variations between samples and batches. Dry thawing systems, with automation and slower thawing rates, help mitigate cell cryoprotectant damage and increase consistency between thawing processes.
What to expect when cells have been thawed
The removal of cells from the thawing system and the washing of cryoprotectant solution (where applicable) mark the end of the cryopreservation cycle. Cryopreservation is a complex process with the potential to strongly impact cells. It is important to consider the effect of time on cell damage post-thawing in order to properly understand the overall biological system impact, as is shown in Figure 3.
Some effects of cryopreservation cycle can be observed immediately, while others take time to develop. This time factor is a key consideration in the widespread use of cell therapies. Cell death following cryopreservation can be stratified by type: necrotic death and apoptosis.
Necrotic death includes both cells that are killed immediately during the cryopreservation process (visible post-thaw as membrane-permeable cells in most cell viability assays) and cells that are totally lost from the system, either through wash-out steps or from complete lysis. These “missing” cells do not usually show up on viability tests as they have been removed from the system, but they are detectable by differences in cell number before freezing and after thawing.35–36
Cell death from apoptosis occurs from several hours up to 2–3 days after thawing (Fig 3), so measuring viability immediately after completion of cryopreservation can yield inaccurate positive results. These cells are sub-lethally damaged and will undergo apoptosis if that damage cannot be repaired. Removing cells from a cell culture environment also lowers their functional and metabolic rates. This phenomenon can become notable post-cryopreservation, so we recommended performing specific functional tests beyond cell number and viability post-thaw.
Trypan blue post-thaw testing is quick, easy, well-established, and cheap to carry out.37 This dye helps determine membrane permeability of cells, which is strongly correlated with cell death; however, cells that are functionally dead can still have an intact membrane at the time the test is performed, leading to false-positive results.36 Trypan blue can also be used for cell counts to establish a true viable cell number.36
Other simple tests include reduction-based assays, in which markers such as fluorescein diacetate, alamarBlue™ reagent, PrestoBlue™ reagent, and MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium) are used to determine the cell metabolic activity.38 These assays are more accurate and less sensitive to false-positives, although they often require an incubation period which adds to the complexity of the process.3
Increasing in complexity are proliferation tests, apoptotic marker tests, and functional assays specific to the cell’s intended use in vivo, such as NK or T cell ability to target a cancer cells expressing a specific marker.35 Ideally, cell viability and activity tests should be performed before patient administration, but this is rarely feasible in the clinic. Therefore, the limitations of immediate viability tests must be clearly understood.
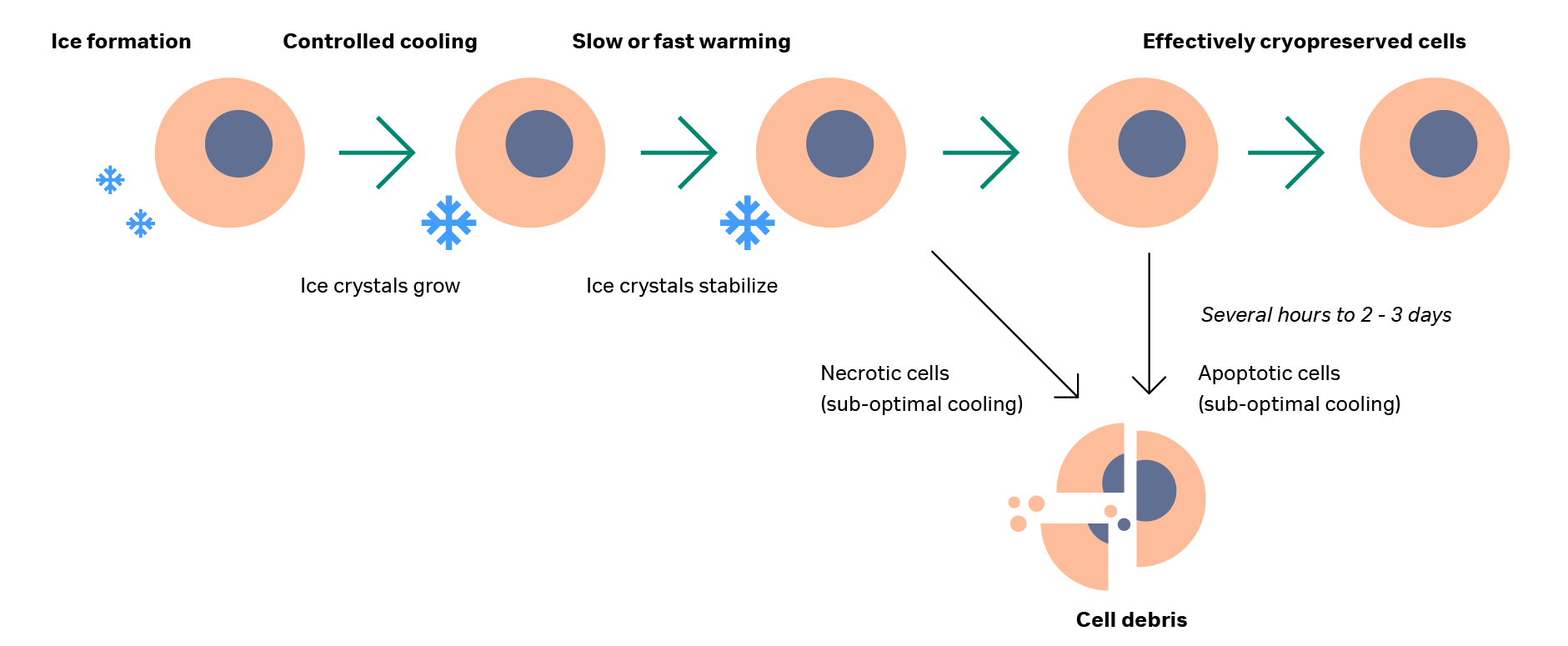
Fig 3. Schematic of cells during cooling, thawing, and after thaw. Ice forms around cells and expands during controlled cooling, remaining stable during both slow and rapid thawing. After thawing, some cells will be damaged and form cell debris while others will undergo apoptosis several days post-thaw. The remainder will survive, having been effectively cryopreserved.
Summary and conclusions
In the past, thawing was the least controlled and consistent step of cryopreservation cycles. Recently, dry thawing systems have become available to reduce problems associated with uncontrolled processes and contamination, delivering consistent and optimized cell therapy thawing.
The science of ice structures and how they change during cooling and warming is also better understood today. Slow, precisely controlled cooling, while essential for cell therapies, also allows for a wider acceptable range of thawing rates.
When assessing viability upon thaw, it is important to ensure that the appropriate assays are applied, taking into account the robustness of these assays as well as treatment practicalities.
Together, these advancements have contributed to better controlled, more precise, and consistent thawing processes, leading to more reliable delivery of effective therapies to patients.
Learn how to simplify and standardize your cell therapy process with cryo solutions from Cytiva
* Including transport while still at ultra-low temperatures, which for DMSO-based systems is usually defined as below -120°C, the glass transition.
- Hunt CJ. Technical considerations in the freezing, low-temperature storage and thawing of stem cells for cellular therapies. Transfusion Medicine and Hemotherapy. 2019;46(3):134-50.
- Fuller BJ, Lane N, Benson EE. Life in the frozen state: CRC press; 2004.
- Meneghel J, Kilbride P, Morris JG, Fonseca F. Physical events occurring during the cryopreservation of immortalized human T cells. PloS one. 2019;14(5):e0217304.
- Baboo J, Kilbride P, Delahaye M, Milne S, Fonseca F, Blanco M, et al. the Impact of Varying Cooling and thawing Rates on the Quality of Cryopreserved Human peripheral Blood t Cells. Scientific reports. 2019;9(1):3417.
- Water-free vs water bath cell thawing. Cytiva App Note. 2019.
- Kilbride P, Meneghel J. Freezing Technology: Control of Freezing, Thawing, and Ice Nucleation. Cryopreservation and Freeze-Drying Protocols: Springer. p. 191-201.
- Ericsson C, Franzén B, Nistér M. Frozen tissue biobanks. Tissue handling, cryopreservation, extraction, and use for proteomic analysis. Acta Oncologica. 2006;45(6):643-61.
- Guest RD, Rothwell DG, Kirillova N, Mowbray S, Sheard V, Gibbons S, et al. 059 Role of cryopreservation in the clinical delivery of T cell based adoptive cell therapies (ACT). Cryobiology. 2013;67(3):414.
- Workflow for primary human NK cell isolation,xeno‑free expansion, harvest, and cryopreservation. Cytiva App Note. 2019.
- Li R, Johnson R, Yu G, Mckenna DH, Hubel A. Preservation of cell-based immunotherapies for clinical trials. Cytotherapy. 2019;21(9):943-57.
- Mfarrej B, Gaude J, Couquiaud J, Calmels B, Chabannon C, Lemarie C. Validation of a flow cytometry-based method to quantify viable lymphocyte subtypes in fresh and cryopreserved hematopoietic cellular products. Cytotherapy. 2020.
- Muyldermans G, De Smet F, Pierard D, Steenssens L, Stevens D, Bougatef A, et al. Neonatal infections with Pseudomonas aeruginosa associated with a water-bath used to thaw fresh frozen plasma. Journal of Hospital Infection. 1998;39(4):309-14.
- Lazarus H, Magalhaes-Silverman M, Fox R, Creger R, Jacobs M. Contamination during in vitro processing of bone marrow for transplantation: clinical significance. Bone marrow transplantation. 1991;7(3):241-6.
- F Lindholm P, Annen K, Ramsey G. Approaches to minimize infection risk in blood banking and transfusion practice. Infectious Disorders-Drug Targets (Formerly Current Drug Targets-Infectious Disorders). 2011;11(1):45-56.
- Röllig C, Babatz J, Wagner I, Maiwald A, Schwarze V, Ehninger G, et al. Thawing of cryopreserved mobilized peripheral blood—comparison between waterbath and dry warming device. Cytotherapy. 2002;4(6):551-5.
- Triana E, Ortega S, Azqueta C, Pomares H, Valdivia E, Duarte R, et al. Thawing of cryopreserved hematopoietic progenitor cells from apheresis with a new dry‐warming device. Transfusion. 2013;53(1):85-90.
- Heger A, Pock K, Römisch J. Thawing of pooled, solvent/detergent-treated plasma octaplasLG®: validation studies using different thawing devices. Transfusion Medicine and Hemotherapy. 2017;44(2):94-8.
- Baust JM, Corwin WL, Snyder KK, Baust JG, Van Buskirk RG. Development and Assessment of a Novel Device for the Controlled, Dry Thawing of Cryopreserved Cell Products. BioProcessing. 2016;15(1):1538-8786.
- Shu Z, Heimfeld S, Huang Z, Liu C, Gao D. Progress in Cryopreservation of Stem Cells and Immune Cells for Cytotherapy. Progress in Stem Cell Transplantation: InTech; 2015.
- Fleck R, Fuller B. 21 Cell Preservation. Medicines from Animal Cell Culture: Wiley; 2007.
- Thompson ML, Kunkel EJ, Ehrhardt RO. Cryopreservation and Thawing of Mammalian Cells: Wiley; 2014.
- Akhtar T, Pegg D, Foreman J. The effect of cooling and warming rates on the survival of cryopreserved L-cells. Cryobiology. 1979;16(5):424-9.
- Kilbride P, Lamb S, Gibbons S, Bundy J, Erro E, Selden C, et al. Cryopreservation and re-culture of a 2.3 litre biomass for use in a bioartificial liver device. PloS one. 2017;12(8):e0183385.
- Drummond NJ, Dolt KS, Canham MA, Kilbride P, Morris GJ, Kunath T. Cryopreservation of midbrain dopaminergic neural cells differentiated from human embryonic stem cells. bioRxiv. 2020.
- Harris L, Griffiths J. Relative effects of cooling and warming rates on mammalian cells during the freeze-thaw cycle. Cryobiology. 1977;14(6):662-9.
- Thorpe P, Knight SC, Farrant J. Optimal conditions for the preservation of mouse lymph node cells in liquid nitrogen using cooling rate techniques. Cryobiology. 1976;13(2):126-33.
- Sahagian ME, Goff HD. Fundamental Aspects of the Freezing. Freezing effects on food quality. 1996;72:1.
- Morris GJ, Goodrich M, Acton E, Fonseca F. The high viscosity encountered during freezing in glycerol solutions: effects on cryopreservation. Cryobiology. 2006;52(3):323-34.
- Morris TJ, Picken A, Sharp DMC, Slater NKH, Hewitt CJ, Coopman K. The effect of Me2SO overexposure during cryopreservation on HOS TE85 and hMSC viability, growth and quality. Cryobiology. 2016;73(3):367-75. doi: https://doi.org/10.1016/j.cryobiol.2016.09.004.
- Elliott GD, Wang S, Fuller BJ. Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology. 2017;76:74-91.
- Barcelo H, Faul J, Crimmins E, Thyagarajan B. A practical cryopreservation and staining protocol for immunophenotyping in population studies. Current protocols in cytometry. 2018;84(1):e35.
- TREE TI, ROEP BO, PEAKMAN M. Enhancing the sensitivity of assays to detect T cell reactivity: the effect of cell separation and cryopreservation media. Annals of the New York Academy of Sciences. 2004;1037(1):26-32.
- Kymriah Data Sheet - ANNEX 1 - SUMMARY OF PRODUCT CHARACTERISTICS. Novartis. 2019.
- Yescarta Data Sheet - ANNEX 1 - SUMMARY OF PRODUCT CHARACTERISTICS. Kite. 2019.
- Baust JG, Gao D, Baust JM. Cryopreservation: An emerging paradigm change. Organogenesis. 2009;5(3):90-6.
- Murray KA, Gibson MI. Post-thaw Culture and Measurement of Total Cell Recovery is Crucial in the Evaluation of New Macromolecular Cryoprotectants. Biomacromolecules. 2020.
- Strober W. Trypan blue exclusion test of cell viability. Current protocols in immunology. 1997;21(1):A. 3B. 1-A. 3B. 2.
- Bahsoun S, Coopman K, Akam EC. The impact of cryopreservation on bone marrow-derived mesenchymal stem cells: a systematic review. Journal of translational medicine. 2019;17(1):397.