Antibody purification
What is antibody purification?
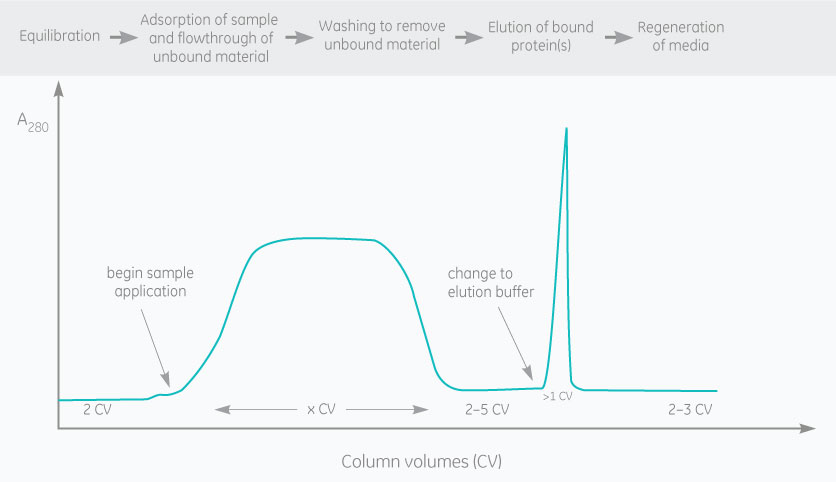
Polyclonal antibodies, monoclonal antibodies (mAb), and antibody fragments are usually purified by affinity chromatography. Resins containing an immobilized ligand (e.g., protein A, protein G or protein L) are used to capture antibodies and antibody fragments.
Affinity purification offers high selectivity. Purity levels above 95% are often possible in just one step. But many uses for antibodies will require further purification. Size exclusion chromatography is often selected for polishing to isolate monomers from aggregates.
How does antibody purification work?
Antibody affinity chromatography is based on the high affinity and specificity of protein A and protein G for the Fc-region of IgG from many species. Protein L, which binds to kappa light chains, is another ligand that can be used to purify antibody fragments, IgG, and other antibodies from a wide range of eukaryotic species.
The binding of an antibody to the ligand is reversible, and the antibody is often eluted by lowering the pH.
When should I use antibody purification?
Affinity chromatography can be used to purify antibodies from cell culture supernatants and serum. Antibody fragments can be purified if they contain the region that interacts with the ligand attached to the matrix. scFv, Fab, and dAb can all be purified using affinity chromatography.
Affinity chromatography can be used as the only purification step for applications that do not require the highest purity. Additional purification steps may be needed based on the intended use.