What is hydrophobic interaction chromatography?
Hydrophobic interaction chromatography (HIC) separates proteins according to differences in their surface hydrophobicity. HIC utilizes a reversible interaction between the proteins and the hydrophobic ligand of a HIC resin.
The interaction between hydrophobic proteins and a HIC resin is greatly influenced by the running buffer. A high salt concentration enhances the interaction. Lowering the salt concentration weakens the interaction.
How does hydrophobic interaction chromatography work?
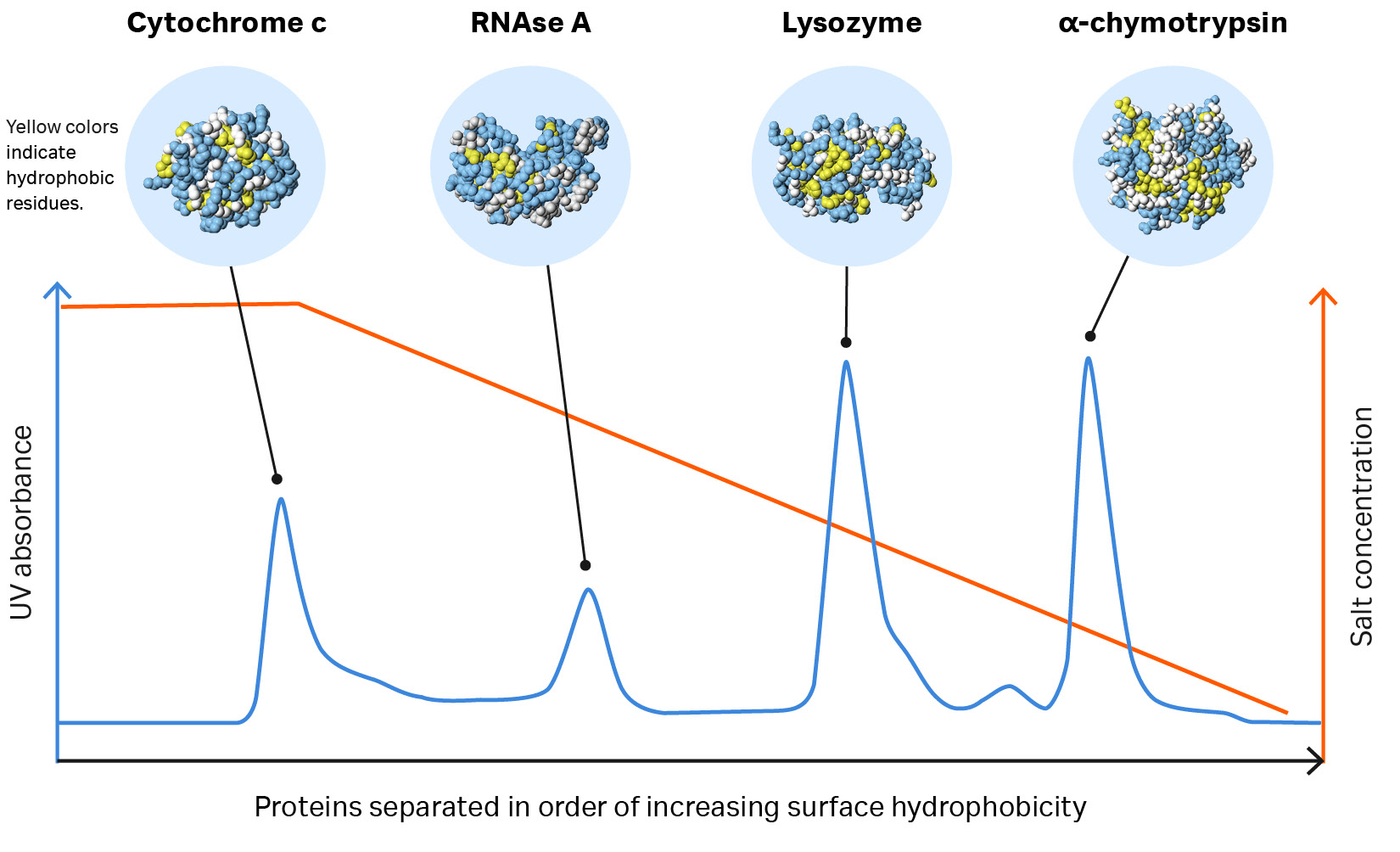
Proteins with different degrees of surface hydrophobicity can be separated using hydrophobic interaction chromatography. The proteins are bound to the hydrophobic ligand on the HIC resin in a binding buffer with a high salt concentration.
When the ionic strength of the buffer is reduced, the interaction is reversed. Therefore, the protein with the lowest degree of hydrophobicity is eluted first. The most hydrophobic protein elutes last, requiring a greater reduction in salt concentration to reverse the interaction. Watch our HIC animation to visualize how HIC works.
When should I use hydrophobic interaction chromatography?
Hydrophobic interaction chromatography can be used for capture, intermediate purification, or polishing steps. Samples should be in a high salt concentration to promote hydrophobic interaction. This means HIC is well-suited for capture steps after sample cleanup by ammonium sulfate precipitation or for intermediate steps directly after an ion exchange separation.
In both situations, the sample is already in a high salt solution. Apart from the addition of more salt, no further preparation is required. As a HIC separation will concentrate the protein of interest into a reduced volume, fractions can also be transferred directly to size exclusion chromatography.
Download this guide that will show you when and why a HIC step is recommended for a powerful purification protocol in research.
How does reversed phase chromatography, RPC, differ from HIC?
Reversed phase chromatography also separates proteins and peptides on the basis of hydrophobicity. Compared with a HIC resin, the surface/ligands of an RPC resin are usually more hydrophobic. This leads to stronger interactions that, for successful elution, must be reversed using non-polar, organic solvents such as acetonitrile or methanol.
As many proteins are denatured by organic solvents, RPC is not generally recommended for protein purification. Instead, RPC is well-suited for applications such as peptide mapping or purity checking.