Make the switch from ELISA to Biacore SPR-based assays
Introduction
Biacore SPR assays provide an automated and reproducible real-time determination of active concentrations that can be considered an alternative to traditional ELISA approach. This article provides general guidance on how to convert an existing ELISA assay to a Biacore SPR-assay and by doing so, shows how you can get results in less than half the time compared with ELISA.
Why Biacore SPR assays and not ELISA?
The principles of concentration or affinity measurements using Biacore systems resemble those applied to automated ELISA. Both are performed on a solid support in which one interaction partner is immobilized (ligand) and the other is free in solution (analyte).
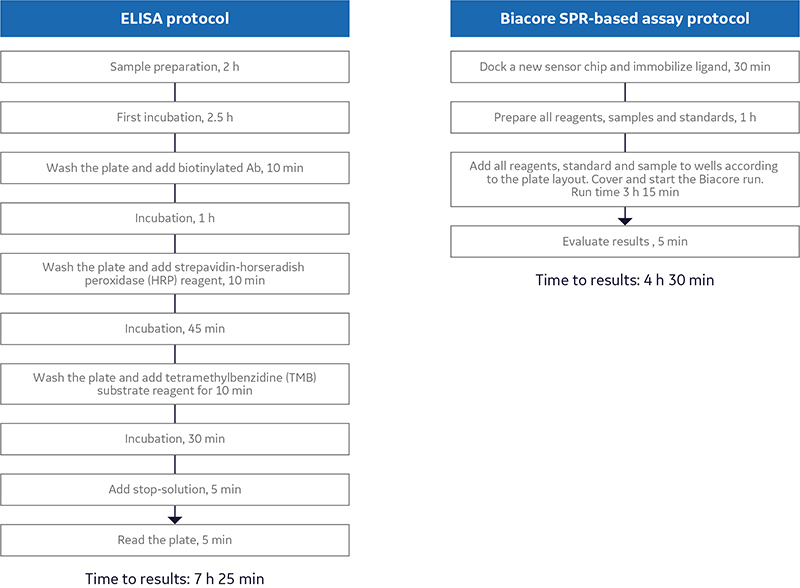
Biacore SPR assays increase operational efficiency by introducing automation and eliminating time- consuming washing steps. The ability to queue several methods and run in sequence to link the surface preparation step to the assay step increases the degree of automation and frees up time. As can be seen in Figure 1, SPR-based assays can be performed in less than half the time compared with ELISA.
Fig 1.(A) A typical ELISA protocol of a 96-well plate with 40 samples (in duplicate) takes 7 h and 30 min.; (B) A typical Biacore SPR assay of 40 samples (in duplicate) is run in 3 h and 15 min (or 4 h and 30 min), providing results in less than half the time compared with ELISA.
Other advantages of Biacore SPR-based assays are:
- Shorter assay development time: flexible assay formats without any labeling of secondary reagents.
- Accurate quantitation and/or affinity analysis of low-affinity/high KD analytes often missed by ELISA.
- Broader selection of assay reagents: SPR enables the use of low-affinity reagents.
- Reduced operating costs: regenerate assay surface to reuse components for additional assays with improved assay reproducibility and robustness allowing fewer reruns.
Key points to consider when developing an SPR assay
In the development of a Biacore SPR assay protocol, the following points need to be considered:
- Selection of assay format (see below)
- Selection of sensor surface, assay buffer, and immobilization procedure
- Development of regeneration conditions, if necessary
- Minimizing nonspecific binding (NSB), if needed
A good starting point before initiating any assay development is to define the minimum requirements for sensitivity, throughput, and total analysis time early in the development process.
Selection of assay format
Depending on assay format, the signal reported in the assay is either directly or inversely proportional to the amount of active analyte bound to the ligand. The conversion of an ELISA to a Biacore SPR assay may involve re-qualification and validation of the conditions and configurations, not unlike the steps required for in development and for optimization of an ELISA method.
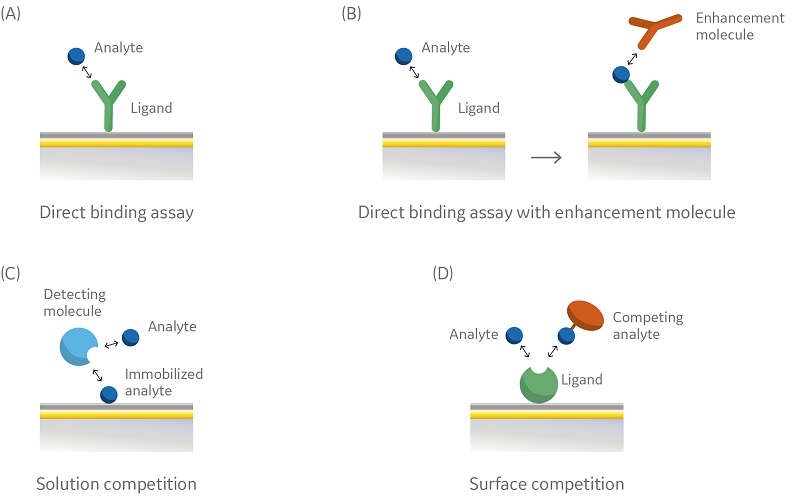
Assay sensitivity, assay range, assay precision, and throughput are important considerations when selecting assay format. Figure 2 (A–D) shows four commonly used SPR assay formats for concentration analysis: A direct binding assay (A) is fast and eliminates the need of a secondary reagent step and several incubations steps. This assay, when used with (B) an enhancement molecule such as a polyclonal antibody, may provide both enhanced sensitivity and specificity.
An indirect assay, such as solution competition (C) and surface competition (D) may also be used as an alternative to direct binding assays in situations where immobilization or regeneration of the ligand is not satisfactory.
Fig 2. Schematic illustration of four different assay formats using Biacore systems: (A) direct binding assay; (B) direct binding assay with enhancement molecule; (C) indirect solution competition assay; (D) indirect surface competition assay.
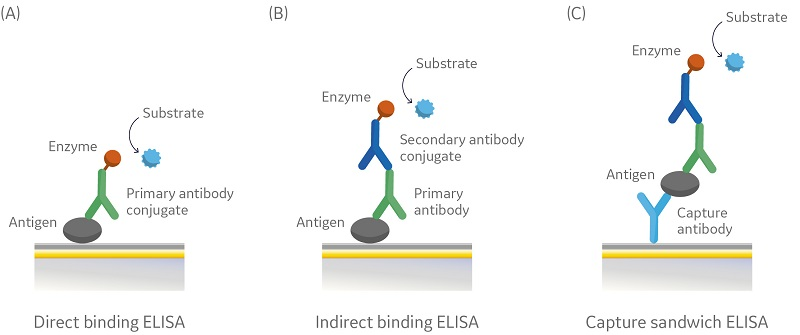
For comparison, Figure 3 illustrates three different commonly used assay formats for ELISA which all require an enzyme-linked secondary reagent for concentration or affinity determinations.
As for ELISA methods, you may increase assay sensitivity in Biacore SPR assays by increasing sample incubation/injection time, which prolongs the analysis time.
Fig 3. Schematic illustration of three different ELISA assay formats: (A) direct binding ELISA; (B) indirect binding ELISA; (C) capture sandwich ELISA.
Selection of sensor surface and immobilization procedure
Generally, the best approach is to covalently immobilize the ligand, which gives a stable sensor surface that can be used repeatedly, provided that the surface can be regenerated between cycles. Amine coupling chemistry through lysine groups is straightforward and works well for many proteins. Coupling through thiol groups is an alternative, and for ligands containing glycosyl residues (with cis-diol groups that can be oxidized to aldehydes), carbohydrazine-based aldehyde coupling can be used.
A broad range of sensor chip surfaces and capture kits to support requirements to determine concentration for a wide range of molecules is available. Development time when working with tagged molecules/ligands or antibody formats can be significantly reduced by using already optimized capture surfaces and reagent kits.
The widely used CM series of sensor surfaces consists of a semi-fluid surface layer of carboxymethylated dextran, which is an excellent choice for covalent immobilization protocols (1).
Sensor Chip SA (pre-immobilized streptavidin) is a ready-to-use surface for capturing of biotinylated ligands and is an alternative to covalent immobilization.
Pre-immobilized sensor chips, such as Sensor Chip Protein A and Sensor Chip Protein G provide an off- the-shelf and ready-to-use solution for determination of antibody concentrations. Sensor Chip Protein L allows determination of antibody fragment concentrations, saving valuable assay development time.
Establishing regeneration conditions
Regeneration is the process of removing bound analyte from the sensor surface after analysis, in preparation for the next analysis cycle. For low-affinity interactions, regeneration of the surface may not be necessary as the analyte often dissociates by itself within a reasonable time, that is, within seconds to a few minutes, saving total run time.
For interactions with higher affinity however, regeneration of the surface is necessary. The number of times a sensor surface can be regenerated depends on the nature of the attached ligand. Antibodies can often be regenerated over hundreds of cycles, and in some cases over a thousand cycles. A selection of ready-to-use regeneration solutions is available from GE to aid scouting for reliable regeneration conditions and capture kits are provided with pre-optimized regeneration conditions, which again saves development time.
Minimizing nonspecific binding (NSB)
Non-analyte components that bind to the surface or to the detecting molecule will contribute to responses in the same way as the analyte. In ELISA assays, surface blocking and wash steps are used to reduce NSB and these steps need to be carefully optimized.
When the sample dilution factor is high enough, the NSB is often negligible in SPR assays. If the analyte concentration is low, further dilution may not be possible and other ways of minimizing NSB may be required. If the NSB is to the dextran surface, adding soluble dextran, that is, an “NSB reducer” to the assay, or including an extra wash injection may significantly reduce the NSB effect.
Selection of another sensor chip during assay development such as Sensor Chip CM4, which has reduced charge density, or Sensor Chip PEG provide further alternatives.
Optimizing buffer conditions, for example by increasing the salt content is another method to reduce NSB. The buffer composition has a significant effect on NSB, signal level, and sample matrix interference. Buffers with low concentrations (~ 10 mM) of HEPES or phosphate at physiological salt concentrations and pH in presence of detergent are a good starting point (2). In many cases a direct transfer of the buffer conditions used in the ELISA is sufficient.
Conclusions
Biacore SPR assays and ELISA assays share many similarities and are based on the same experimental principles. The conversion of an ELISA-assay into a Biacore SPR-assay may only require the transfer of the antibody pairs used in the ELISA and the assigned buffer conditions.
The advantages of using Biacore SPR assays include:
- Results are obtained in less than half the time compared with ELISA.
- Very low operating expenses thanks to the automated setup.
- The ability to reuse of sensor surface significantly reduces consumables expenses.
- Low sample consumption and high reproducibility that minimizes the need for assay reruns
- Easy-to-use: no biotinylated antibodies, streptavidin-HRP reagent and other developing reagents nor wash buffer preparation and repetitive washing steps are needed.
- Automation that not only frees up time for another analytical task but also decreases inter-and intraassay variability.
- Suitable for more complex assays, such as bispecific antibodies.
- SPR assays not prone to edge effects or the hook effect as in ELISA assays.
References
- Navratilova, I. et al. Analyzing ligand and small molecule binding activity of solubilized GPCRs using biosensor technology. Anal. Biochem. 355, 132–139 (2006). DOI: 10.1016/j.ab.2006.04.021
- Moberg, A. et al. Increased sensitivity of SPR assays in plasma through efficient parallel assay optimization. J. Pharm. Biomed. Anal. 5(78–79), 224–232 (2013). DOI: 10.1016/j.jpba.2013.02.018