To View Part one of this series, click here.
What is chemiluminescence-based detection?
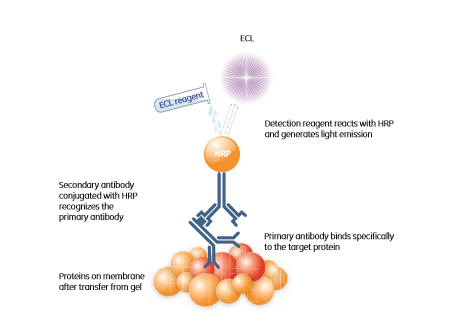
Chemiluminescence detection occurs when a chemical reagent containing stored energy releases light. The reagent is normally stable and does not emit light, but can be converted into a light-emitting product, for example, after interaction with a specific enzyme. In most contemporary ECL (emitter coupled logic) systems, the enzyme horseradish peroxidase (HRP) conjugated to a secondary antibody is the catalyst that fulfils this function (Fig 1.5).
The light produced is proportional to the amount of labelled compound in the sample and can be detected on X-ray films as well as by using CCD camera-based imagers (charge coupled device). ECL is more versatile than general colorimetric methods as these antibody-based systems are usually designed to target specific biomolecules. In addition, the technique is fast and sensitive; signals are generated in seconds and relatively small quantities of antigens and antibodies are normally consumed. As light is generated without an external excitation source, there is no risk of photodamage to samples.
Fig 1.5: Chemiluminescence-based protein detection. The HRP conjugated secondary antibody will recognize the primary antibody directed against the protein of interest. This enzyme catalyzes the conversion of the ECL substrate into a sensitized reagent, which on further oxidation produces an excited state that emits light (428 nm) when it decays.
Sensitivity and precision
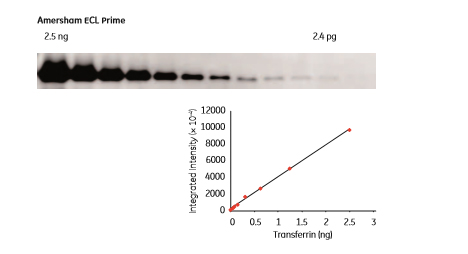
The most recent ECL reagents give rise to high sensitivity and linearity of signal response over a wide range of protein levels. The results shown in Figure 1.6 were obtained after 75 s exposure using Amersham™ ECL™ Prime, and enable detection and precise quantitation of both high and low abundant proteins on the same blot after a single exposure.
Fig 1.6: Western blotting detection of transferrin in a 2-fold dilution series using Amersham ECL Prime. The LOD was 2.4 pg transferrin and the linear dynamic range was 3.0 orders of magnitude. The blot was imaged using a CCD-based ImageQuant™ LAS 4000 mini system.
Signal stability
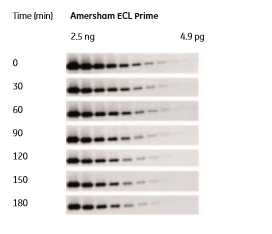
The light signal emitted by an ECL reagent reaches its maximum intensity shortly after activation and then diminishes with time. The rate of signal quenching varies among the available reagents but usually lies in the range of a few minutes to 2 h. The chemiluminescent signal produced by Amersham ECL Prime is highly stable, with measureable signals remaining up to 3 h after starting the reaction between HRP and the luminescent reagent, even for the lowest amounts of protein tested. This enables multiple exposures and a convenient time window between the end of the experiment and image capture (Fig 1.7).
Fig 1.7: Signal intensities 5 min after Amersham ECL Prime reagent addition and at time intervals up to 3 h after reagent addition. Image exposure was set at 3 min for all the samples.
Fluorescence detector vs chemiluminescence-based detection
Fluorescence detection is replacing chemiluminescence-based detection due to improved sensitivity, the development of new fluorescent labels and the reduced cost of fluorescent detection systems. Fluorescent detection also opens the possibility of multiplexing, where samples containing differentially labelled proteins can be simultaneously detected on a single gel or blot.
The type of imager to use depends heavily upon the detection system or systems you choose and the quality and quantity of data required from your experiments. Densiometers, provide high resolution densiometry for X-ray films exposed to radioisotopes or light and gels stained with reagents such as Coomassie Blue or silver. With CCD camera-based systems, such as the ImageQuant LAS series, it is possible to cover a full range of tasks, from sample documentation to fluorescence and chemiluminescence. For maximum versatility in imaging, high performance variable mode imagers are available, such as those in the Typhoon™ FLA series. These imagers make it possible to handle phosphorimaging, multiplex fluorescence, and chemifluorescence with high throughput, while maintaining quantitative precision and low detection limits.
Other detection systems
Chemifluorescence refers to the enzymatic production of fluorescence. As with chemiluminescence, a substrate is needed. A catalytic reaction between enzyme and substrate results in the formation of a fluorescent product at the site of the reaction, as well as an amplification of the signal. The newly generated fluorescent product can be induced to emit light, based on the same excitation/emission principle described for fluorescence. The signal can be detected by using a suitably equipped CCD camera-based imaging system or laser scanner. Colorimetric methods using dyes such as Coomassie Blue and silver staining are still widely used for visualization and detection of proteins. Silver is about 10- to 100-fold more sensitive than Coomassie Blue and can be used to detect as little as sub-nanogram quantities of protein. Silver staining is therefore the method of choice where sensitivity is more important than accurate quantitation, due to a relatively narrow dynamic range of detection.
Staining with Coomassie Blue is simpler than silver staining and is reasonably sensitive. Images of lanes of blue bands are universally recognized among researchers as resolved proteins on polyacrylamide gels. Coomassie Blue reliably detects protein in the 5 to 500 ng range, but staining and destaining usually takes at least 2 h and demands large quantities of noxious solvents. In addition, Coomassie Blue binds proteins with variable affinity; glycoproteins, for example, which make up more than half the total complement of proteins, stain poorly with Coomassie Blue.
Although alternatives to radioisotope-based detection systems have emerged, there are still some areas in which radioisotopes continue to offer some advantages. Radioactive systems in combination with phosphorimaging screens are still used in Southern and Northern blotting because of their sensitivity and speed. Phosphorimaging screens are sensitive to any source of ionizing radiation, including commonly used isotopes such as 32P, 33P, 35S, 14C, 3H and 125I. They generate signals across a linear dynamic range of up to five orders of magnitude, allowing precise quantitation of weak and strong signals in one exposure.