Principle of protein electrotransfer
In Western blotting, protein electrotransfer, or electrophoretic transfer, uses the same protein mobility principles as the preceding polyacrylamide gel electrophoresis (PAGE, Fig 1.)
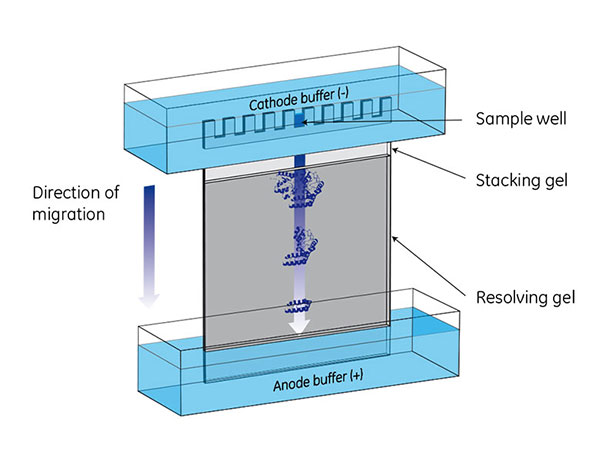
Fig 1. A vertical electrophoresis apparatus is set up with cathode (-) buffer in an upper chamber and anode (+) buffer in a lower chamber. Molecules in sample wells migrate toward the anode (+) in an electric field. After a set amount of time, the molecules will have migrated to positions based on their size, shape, and charge.
PAGE separates proteins according to size, producing distinct bands on the gel when stained. In the presence of an electric field, protein mobility relies on having a net negative charge, which moves the proteins towards the anode.
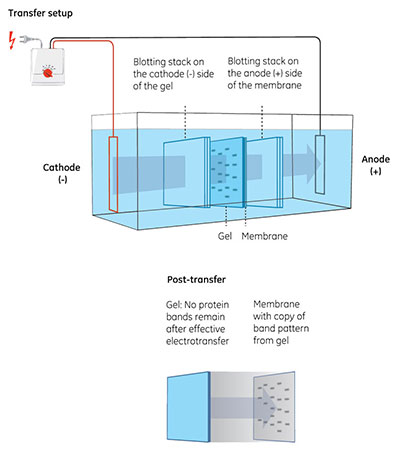
In the subsequent electrotransfer, the proteins are migrated to a membrane where electrostatic and hydrophobic forces immobilize them (Fig 2).
Understanding the underlying principles of protein transfer, and how various components of the Western blot setup affect this, can increase transfer success.
Fig 2. Transfer of proteins from gel to membrane. The gel is placed in contact with the membrane and the proteins then migrate toward the positively charged anode (+) in an electric field.
How the electric field affects protein transfer
The electric field is fundamental for successful protein transfer. Conducted via a transfer buffer, the field is responsible for pulling negatively charged proteins out of the gel and onto the membrane.
This field is dependent on Ohm’s law, which relates voltage (V), current (I), and resistance (R) as V = I × R. Using a constant transfer voltage maintains the field strength throughout, providing efficient blotting.
However, power from the electric field dissipates as heat in the buffer, reducing the buffer’s resistance. This effect leads to transfer systems increasing current to maintain the voltage, potentially affecting transfer efficiency.
Maintaining a constant transfer current produces a gradually decreasing field strength, as transfer systems reduce voltage to account for decreasing buffer resistance. This effect reduces the efficiency of blotting, adding more time to the transfer.
Conducting transfers at 4°C or with active cooling will minimize unwanted buffer warming and its associated issues.
Considerations for transfer buffers
The transfer buffer provides a conductive medium for transfer. The buffer maintains a stable pH and supports both elution of proteins from the gel and binding to the membrane.
Common general purpose buffer formulations include Tris/Glycine, and CAPS. Each buffer formulation varies in pH and might contain a surfactant and solvent, depending on the mobility and binding affinity of target proteins.
During transfer, the buffer gradually warms, breaking down and losing its buffering capacity and electrical resistance, which means buffers are only suitable for a single use.
Surfactant
The surfactant sodium dodecyl sulfate (SDS) is commonly used during PAGE to bind and denature proteins, producing the net negative charge for protein electromobility.
However, SDS is less desirable for transfer as it lowers a protein’s binding affinity, particularly for nitrocellulose membranes. At the same time, SDS is required for protein mobility out of the gel.
To achieve a balance, washing the gel before protein blotting removes most of the SDS while adding a small percentage to the transfer buffer allows for both protein mobility and binding. The optimal percentage of SDS requires testing for the protein of interest, but is generally in the region of 0.05% (w/v).
Solvent
Adding an organic solvent to the transfer buffer, most often methanol, improves protein binding by stripping SDS from proteins and preventing further interaction. This action particularly benefits binding to NC membranes.
However, a high percentage of methanol can dehydrate the gel, reducing the pore sizes and precipitating some proteins, affecting transfer efficiency. As with SDS, achieving a balance minimizes these negative effects.
Methanol at 20% (v/v) is common to transfer protocols, but reducing this to 10% (v/v) can improve elution of large proteins from the gel.
Western blot membranes
The membrane is part of the sandwich—the assembly of gel, membrane, and filter papers—used in electrotransfer. Placing the membrane closest to the anode in this setup makes sure it can capture and bind the proteins as they move out of the gel.
Membranes bind proteins through hydrophobic and electrostatic interactions. The most commonly used membrane materials are nitrocellulose ([NC], with or without a polyester support) and polyvinylidene difluoride (PVDF). The differing properties of these materials affect the choice of membrane.
NC membranes bind proteins immediately and almost irreversibly. PVDF provides a high binding efficiency and mechanical strength, but requires wetting with an organic solvent such as methanol before use. Both materials support various protein detection methods to suit different experiments.
More information about efficient transfer can be found in the Western blotting handbook.