Size-exclusion chromatography (SEC) is often used in the downstream processing of large and sensitive entities, such as viruses or extracellular vesicles (EVs or exosomes). But this SEC step is often seen as a productivity bottleneck — low flow rates and limited sample loads can slow virus and exosome purification. Capto™ Core beads were developed to address this bottleneck and intensify downstream processing of large entities. The core bead technology includes a chromatography resin with ligand‑activated core and an outer inactive shell (Fig. 1). This structure enables dual functionality, in which smaller impurities that can diffuse through the shell are captured, while larger target entities are collected in the flowthrough1. By combining size separation and binding chromatography, Capto™ Core resins can be a good alternative to SEC resins for purification of exosomes or other large entities.
Fig 1. Capto™ Core bead technology includes a chromatography resin with ligand‑activated core and an outer inactive shell.
Core bead functionality
The inert shell combined with ligand‑activated core enables high sample load in combination with group separation of molecules. The core of each bead is functionalized with ligands that are both hydrophobic and positively charged, resulting in a highly efficient multimodal binding of various impurities small enough to diffuse through the shell and enter the core. The multimodal ligands ensure strong binding with most impurities over a wide range of pH and salt concentrations and limited diffusion of impurities out from the bead1. This is the beauty of core bead technology: it allows processing of large sample volumes using a relatively small chromatography column. In essence, you can use these core beads to scavenge, or cut away, small impurities from large and sensitive target entities, like virus capsids, enveloped viruses, or exosomes.
The shell of the core bead does not have mono-sized pores. Instead, it has a pore size distribution, with an average cutoff corresponding to a 700 kDa globular entity for Capto™ Core 700 and a 400 kDa globular entity for Capto™ Core 400 resin. These perceived cutoff numbers are valid at 3 min residence time, corresponding to 400 cm/h in a column with 20 cm bed height1. The perceived cutoff is influenced by flow rate. With lower flow rate, there is greater likelihood that large entities will pass through the shell and bind the ligand in the core. Thus, lowering the flow rate lowers recovery of large-sized target entities such as viruses or vesicles but improves impurity removal (Fig. 2A). Increasing the flow rate improves recovery of large-sized target entities at the cost of impurity reduction. The correlation between flow rate and yield is shown in Figure 2B.
Fig 2. Target molecule recovery percentages of Capto™ Core beads for different viruses (A) and at different flow rates (B).
Core beads for exosome purification
Exosomes are not a homogenous group of vesicles. They include subpopulations with different sizes and biological properties. In terms of size, exosomes also overlap to various extents with other EVs, namely microvesicles and apoptotic bodies2,3. Core bead technology is useful to gently purify a distinct subpopulation of EVs.
You can remove undesired large vesicles with standard midstream filtration technologies such as normal flow filtration or depth filtration, complemented as needed with slow-speed centrifugation. This primary clarification removes cell debris and vesicles larger than your target EV subpopulation. You can then use core bead technology to scavenge impurities smaller than the target EV subpopulation, without need to concentrate the feed4-6. Optimized primary clarification in combination with a core bead flow-through chromatography step is often an attractive and gentle downstream workflow for exosome purification. To successfully remove small impurities without compromising recovery, it is important to choose the appropriate type of core bead and fine tune the core bead flow-through chromatography step.
You can also, if needed, associate core bead technology with a bind/elute step to remove similarly sized EVs — like apoptotic bodies and microvesicles — from your target subpopulation of exosomes6. You can design this bind/elute step to fit existing chromatography systems.
Fig 3. Gentle exosome production workflow without bind/elute step.
Case study: recovery and purity with different Capto™ Core resins
Dr Ryan McNamara and Prof Dirk Dittmer at the University of North Carolina evaluated the relationship between recovery and purity for Capto™ Core 400 and 700 resins. They used 1 mL HiTrap™ columns prepacked with Capto™ Core 700 or Capto™ Core 400 resin as a purification step in an exosome isolation process after tangential flow filtration (TFF) and PEG precipitation4. Purity was measured as number of exosome particles per microgram protein in this study. Exosomes of 30–150 nm are larger than the cutoff for both resins. The cutoff for Capto™ Core 400 is 400 kDa and for Capto™ Core 700 is 700 kDa. The assumption would be that exosomes will come in the flowthrough whereas the protein binds to the Capto™ Core beads (this can be regulated with flow rate, as described above). So which Capto™ Core bead should be used in the process?
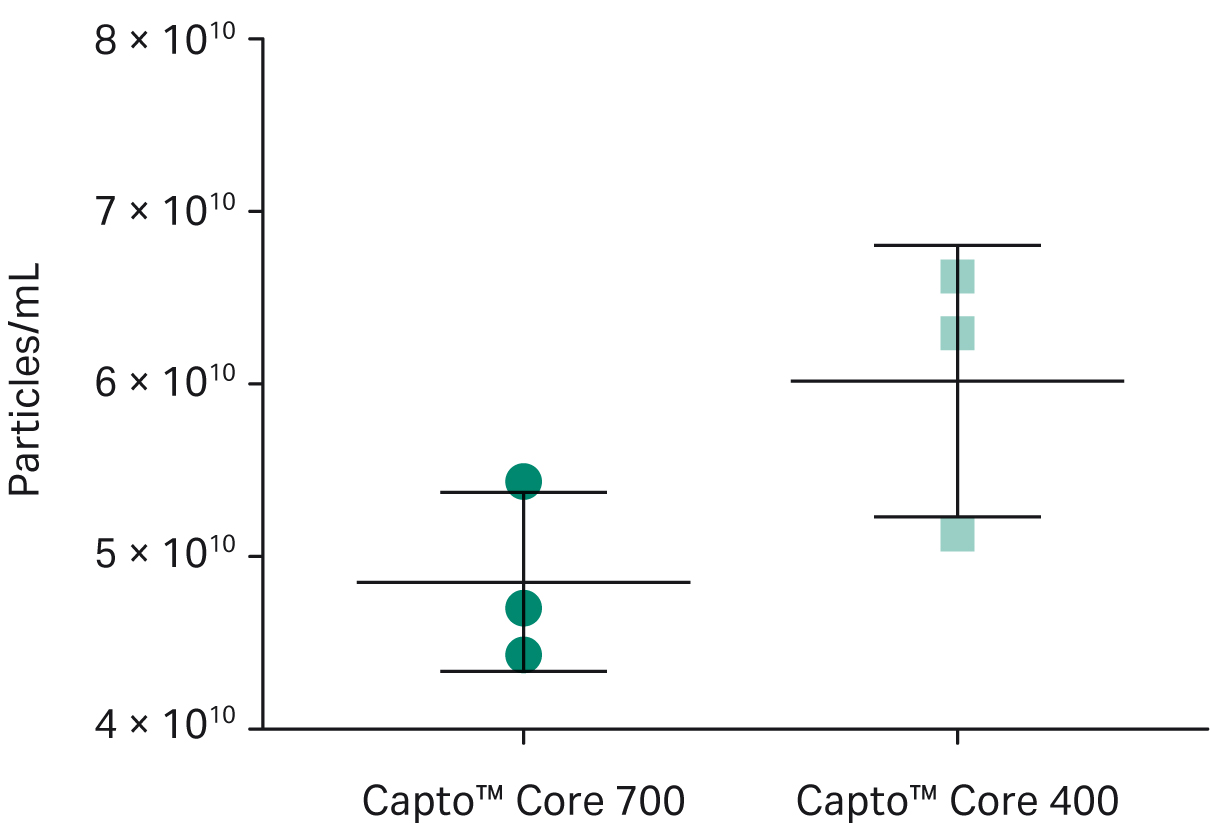
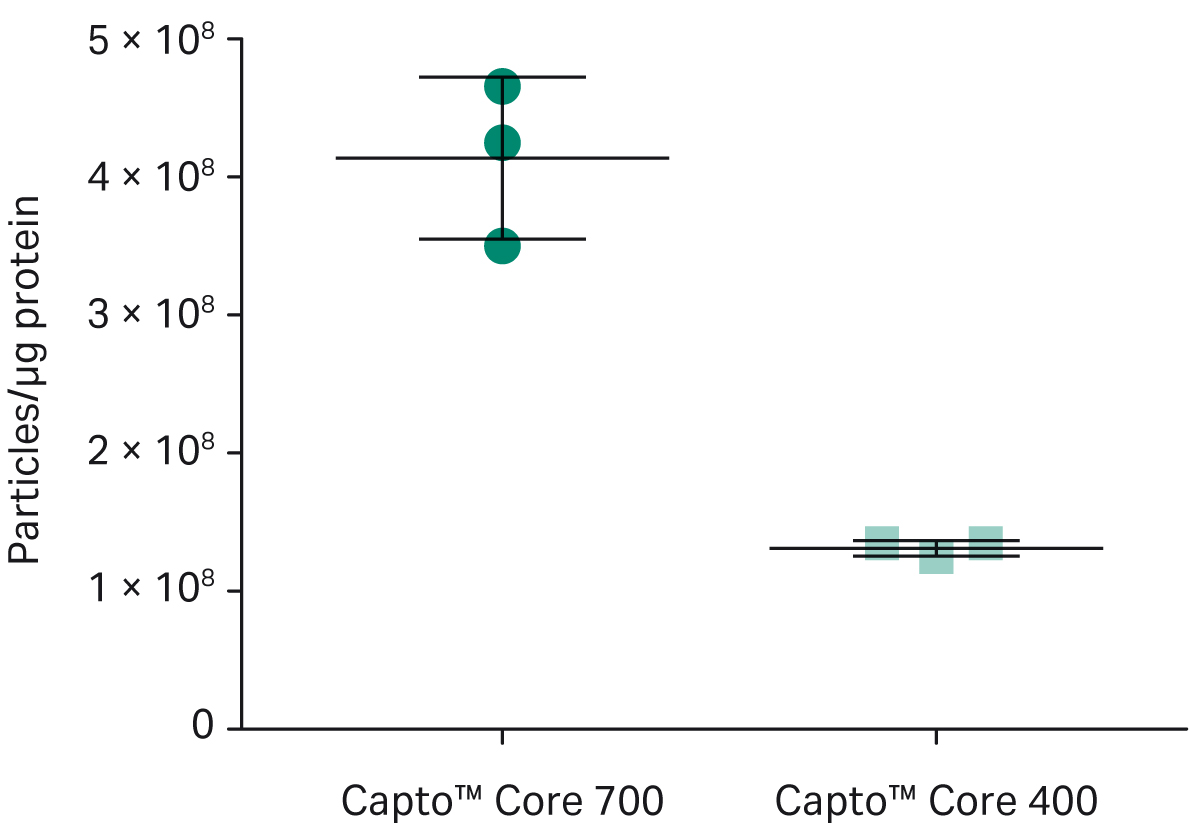
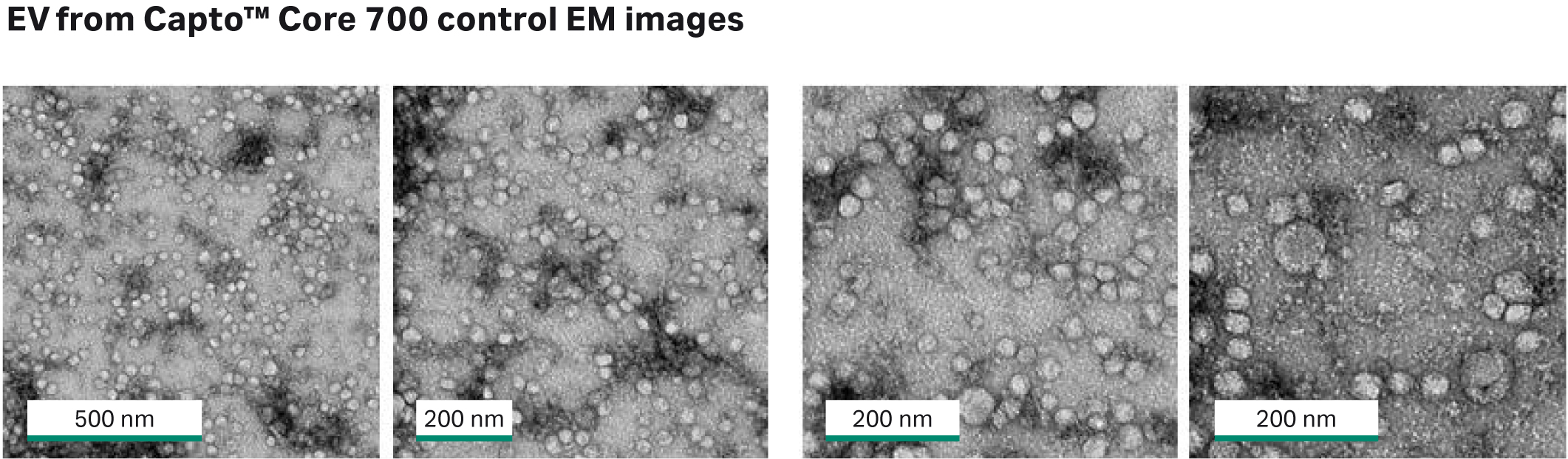
As shown in Figure 4, exosome recovery was higher with Capto™ Core 400 (final exosome mean concentrations were 5.4 ×1010 particles/mL for Capto™ Core 700 resin and 6.6 × 1010 particles/mL for Capto™ Core 400 resin). However, as shown in Figure 5, more protein was removed with Capto™ Core 700 resin (4 × 108 exosome particles/μg protein with Capto™ Core 700 resin vs 1.5 × 108 exosome particles/μg protein for Capto™ Core 400 resin). Electron microscopy4,5 (Fig. 6) showed that exosomes were still intact after purification with Capto™ Core 700 beads. These results suggest that Capto™ Core 700 beads should be used when purity is a major factor. A potential drawback with the superior impurity removal of Capto™ Core 700 is the recovery of exosome particles. Variation in the number of exosomes passing the shell of Capto™ Core 700, i.e., exosome recovery, contributes to higher experimental variation for Capto™ Core 700 as compared to Capto™ Core 400 in Figure 5. The number of exosomes passing the shell of Capto™ Core 400, i.e., loss in recovery, is very limited.
Fig 4. Particle recovery with Capto™ Core 700 beads and Capto™ Core 400 beads. Recovery was higher for Capto™ Core 400 beads, at 6.6 × 1010 particles/mL, vs 5.4 × 1010 for Capto™ Core 700 beads. Flow rate was equivalent for both resins. All runs were performed on an ÄKTA start™ chromatography system. Fractions from each flowthrough were evaluated using a Particle Metrix ZetaView™ nanoparticle tracking analyzer and validated with quantitative immunoblotting of the tetraspanin proteins CD63, CD9, and CD81.
Fig 5. Product purity after exosome purification using Capto™ Core 400 and Capto™ Core 700 beads. Product purity was better with Capto™ Core 700 beads: 4 ×108 particles/μg protein vs 1. 5 ×108 exosome particles/μg protein for Capto™ Core 400 beads. The protein content was analyzed with BCA assay. All runs were performed on an ÄKTA start™ chromatography system. Fractions from each flowthrough were evaluated using a Particle Metrix ZetaView™ nanoparticle tracking analyzer and validated with quantitative immunoblotting of the tetraspanin proteins CD63, CD9, and CD81.
Fig 6. Electron microscopy images from flowthrough fraction after purification with Capto™ Core 700 beads. The exosomes are intact after purification.
Summary
Large and sensitive entities, such as viruses or extracellular vesicles, can be purified in scalable manner with good impurity removal and high recovery. Core beads allow for gentle, quick, and efficient impurity removal in flow-through mode. Optimization of running parameters (flow) allow for selecting a desired trade-off between recovery and impurity removal for large entities, like exosomes, of various sizes and with different impurity profiles.
Acknowledgements
We kindly acknowledge Dr Ryan McNamara and Prof Dirk Dittmer at University of North Carolina for evaluating Capto™ Core 400 and 700 resins with their exosome feed and for granting us permission to publish the data.
LEARN MORE ABOUT CAPTO™ CHROMATOGRAPHY RESINS- Cytiva. Multimodal chromatography Capto Core 400 Capto Core 700 Data File. Capto Core 400 and Capto Core 700 - Multimodal chromatography resins (cytivalifesciences.com)
- McNamara, RP, Dittmer, DP Extracellular vesicles in virus infection and pathogenesis. Curr Opin Virol. 2020; 44: 129-138.
- Willms E, Johansson HJ, Mäger I, Lee Y, Blomberg KEM, Sadik M, Alaarg A, Smith CIE, Lehtiö J, EL Andaloussi S, Wood MJA, Vader P. Cells release subpopulations of exosomes with distinct molecular and biological properties. Nat Sci Reports. 2016; 6:22519
- McNamara RP, Caro-Vegas CP, Costantini LM, Landis JT, Griffith JD, Damania BA et al. Large-scale, cross-flow based isolation of highly pure and endocytosis-competent extracellular vesicles. J Extracell Vesicles. 2018; 7(1): 1541396.
- McNamara RP, Dittmer, DP Modern techniques for the isolation of extracellular vesicles and viruses. J Neuroimmune Pharmacol. 2020; Sep 15(3): 459–472.
- Guterstam P, Whitford W.Exosome manufacturing status. Future Medicinal Chemistry. 2019: (10):1225-1236
Learn more about Capto™ Core resins, ÄKTA™ chromatography systems and production of exosomes:
- Capto Core 700 multimodal chromatography resin
- Chromatography systems
- Develop and refine your AAV production process
- Manufacturing of viral vectors
- Improving lentiviral vector downstream processing workflows
- Purification of infectious virus particles using ÄKTA start
- FAQs: AAV production and purification
- Accelerate flavivirus vaccine production
- Process development challenges for mRNA vaccines and beyond